How many pi bonds in a triple bond
Post by » Sat Dec 04, pm. Laurence Lavelle Skip to content. Quick links. Email Link.
Sigma and pi bonds are types of covalent bonds that differ in the overlapping of atomic orbitals. Covalent bonds are formed by the overlapping of atomic orbitals. Sigma bonds are a result of the head-to-head overlapping of atomic orbitals whereas pi bonds are formed by the lateral overlap of two atomic orbitals. Various bond parameters such as bond length, bond angle, and bond enthalpy depend on the way the overlapping of atomic orbital takes place. This overlap occurs in two major ways, giving rise to two primary types of covalent bonds , i.
How many pi bonds in a triple bond
Our minds can handle two electrons interacting with one another in a sphere of space. But then we start putting in double bonds and triple bonds. So we need a more complex picture that works for all these electrons. The hybridization model helps explain molecules with double or triple bonds see figure below. The entire molecule is planar. As can be seen in the figure below, the electron domain geometry around each carbon independently is trigonal planar. Each contains one electron and so is capable of forming a covalent bond. Three sigma bonds are formed from each carbon atom for a total of six sigma bonds in the molecule. The pi bond is the "second" bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. This plane contains the six atoms and all of the sigma bonds. It is important to realize, however, that the two bonds are different: one is a sigma bond, while the other is a pi bond. The promotion of an electron in the carbon atom occurs in the same way.
Valency Of All Elements. Who is online Users browsing this forum: No registered users and 0 guests.
.
Forgot password? New user? Sign up. Existing user? Log in. Already have an account? Log in here. Sigma and pi bonds are chemical covalent bonds. Sigma and pi bonds are formed by the overlap of atomic orbitals.
How many pi bonds in a triple bond
Our minds can handle two electrons interacting with one another in a sphere of space. But then we start putting in double bonds and triple bonds. So we need a more complex picture that works for all these electrons. The hybridization model helps explain molecules with double or triple bonds see figure below. The entire molecule is planar. As can be seen in the figure below, the electron domain geometry around each carbon independently is trigonal planar. Each contains one electron and so is capable of forming a covalent bond. Three sigma bonds are formed from each carbon atom for a total of six sigma bonds in the molecule. The pi bond is the "second" bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule.
Rüyada otobüs bileti almak diyanet
The orientation of the two pi bonds is that they are perpendicular to one another see figure below. Copper Atomic Number. Start Quiz. Sign in. Aldol Condensation. But then we start putting in double bonds and triple bonds. The three most common overlap conditions that result in sigma bonds are:. What Are Oxides. The benzene ring consists of six carbon-carbon single bonds, all of which are sigma bonds. The pi bond is the "second" bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule.
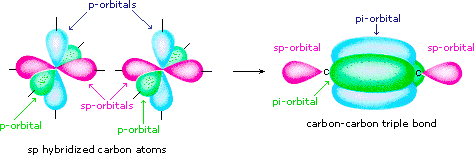
The hybrid orbital model appears to account well for the geometry of molecules involving single covalent bonds. Is it also capable of describing molecules containing double and triple bonds?
This type of covalent bonding is illustrated below. Additionally, there exist six carbon-hydrogen sigma bonds. C and N are triple bonded and each atom has one lone pair. Valency Of All Elements. Hydrochloric Acid Formula. Therefore, the total number of pi bonds in a benzene molecule is 3. Download Now. So we need a more complex picture that works for all these electrons. The promotion of an electron in the carbon atom occurs in the same way. A triple bond consists of two pi bonds and one sigma bond.

Your idea is very good
In it something is also idea good, I support.