Charge of io3
The Charge of IO3 Iodate ion is But the question is how can you find the charge on IO3 iodate ion? So you can easily say that the charge of IO 3 should be 1- then only it will get canceled out, charge of io3. So here also you can easily say that the charge of IO 3 should be 1- then only it will get canceled out.
Iodate ion contains one iodine and three oxygen atoms. There is -1 charge on oxygen atom in IO 3 - lewis structure. Oxygen atoms have made bonds with center iodine atom. From those bonds, there are two double bonds and one single bond in the IO 3 - lewis structure. Also, there is one lone pair exist on iodine atom -1 charge exists on one oxygen atom in the lewis structure of IO 3 - ion. When we draw a lewis structure, there are several guidelines to follow.
Charge of io3
IO 3 — lewis structure has an Iodine atom I at the center which is surrounded by three Oxygen atoms O. There is 1 single bond and 2 double bonds between the Iodine atom I and each Oxygen atom O. There is 1 lone pair on Iodine atom I , 2 lone pairs on double bonded Oxygen atom O and 3 lone pairs on single bonded Oxygen atom O. In order to find the total valence electrons in IO3- ion, first of all you should know the valence electrons present in iodine atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Iodine is a group 17 element on the periodic table. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of iodine atom I and oxygen atom O in the above periodic table. If we compare the electronegativity values of iodine I and oxygen O then the iodine atom is less electronegative. Now in the IO3 molecule, you have to put the electron pairs between the iodine atom I and oxygen atoms O. This indicates that the iodine I and oxygen O are chemically bonded with each other in a IO3 molecule. Also, in step 1 we have calculated the total number of valence electrons present in the IO3- ion.
To do that, we can form a double bond with the Oxygen by moving these valence electrons and share them with the Iodine.
Wiki User. Rubidium iodate. Formula: NaIO3. Pt IO3 8. Formula: CuIO3. The chemical formula of iodate is IO
So far, we have discussed elements and compounds that are electrically neutral. They have the same number of electrons as protons, so the negative charges of the electrons are balanced by the positive charges of the protons. However, this is not always the case. Electrons can move from one atom to another; when they do, species with overall electric charges are formed. Such species are called ions. Species with overall positive charges are termed cations , while species with overall negative charges are called anions. Remember that ions are formed only when electrons move from one atom to another; a proton never moves from one atom to another. Compounds formed from positive and negative ions are ionic compounds.
Charge of io3
It is the most common form of iodine in nature, as it comprises the major iodine-containing ores. They are the salts of iodic acid. Iodate is pyramidal in structure. It participates in several redox reactions, such as the iodine clock reaction. Iodate shows no tendency to disproportionate to periodate and iodide, in contrast to the situation for chlorate.
Frases de un sabio dijo sobre el amor
Iodate ion contains one iodine and three oxygen atoms. L Lanthanum Iodate. Number of steps can be changed according the complexity of the molecule or ion. From those bonds, there are two double bonds and one single bond in the IO 3 - lewis structure. There is 1 lone pair on Iodine atom I , 2 lone pairs on double bonded Oxygen atom O and 3 lone pairs on single bonded Oxygen atom O. Your email address will not be published. Amphoteric nature of water NO 2 - lewis structure N 2 O lewis structure, resonance structures Stability of water. Chemical formula. Formula: NaIO3. Oxygen is a group 16 element on the periodic table. Follow Us. In the above lewis dot structure of IO3- ion, you can also represent each bonding electron pair : as a single bond.
IO 3 — iodate has one iodine atom and three oxygen atoms. In IO 3 — Lewis structure, there are two double bonds and one single bond around the iodine atom, with three oxygen atoms attached to it.
Read more about our Editorial process. To do that, we can form a double bond with the Oxygen by moving these valence electrons and share them with the Iodine. I am sure you will definitely learn how to draw lewis structure of IO In these compounds, an ionic bond is formed between a metal cation and the iodate anion, which consists of one atom of iodine covalently bound to three atoms of oxygen, and carries a formal charge of Also, there is one lone pair exist on iodine atom -1 charge exists on one oxygen atom in the lewis structure of IO 3 - ion. Let me explain the above image in short. Now, we know how many electrons are there in valence shells of oxygen and iodine atoms. We have 6, 8, 10, 24; and then back to the central Iodine, Oxygen is a group VIA element in the periodic table and has six electrons in its last shell valence shell. Space-filling model of the iodate anion. Number of steps can be changed according the complexity of the molecule or ion.

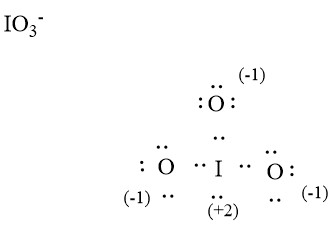
It is removed (has mixed topic)
This topic is simply matchless