Is kbr ionic or molecular
Potassium bromide K Br is a saltwidely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to in the US.
In this content, you will find all important information about potassium bromide uses, its properties, and production. Potassium bromide is a chemical compound of the element potassium or K and bromine or Br 2. At room temperature, potassium reacts with bromine, and by synthesis, this compound is formed. Potassium bromide has an immense contribution to medical science. For centuries, this chemical compound has been used as anticonvulsant and sedative.
Is kbr ionic or molecular
Potassium K is a chemical element and its atomic number is Potassium is a silvery-white metal that sifts enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. Br is the compound name of Bromine and is an essential element of the periodic table. The bromine substance Br 2 is a rosy earthy colored fluid natural d liquid and is never ordinarily found in its basic structure design yet rather in inorganic combinations, called bromides and in organobromine compounds. These are ordinarily found in soils, salts, air, and seawater. Potassium bromide is a chemical compound that consists of the elements potassium K and bromine Br. At room temperature, by process of synthesis, potassium reacts with bromine, and potassium bromide is formed. It is used as an anticonvulsant and sedative. It is also known as Kali bromidum, tripotassium tribromide, and bromide salt of potassium. It is a colorless crystalline Powder. This compound is completely soluble in water, when put into water, it can be quickly disassociated into individual ions and disappear. The formula for potassium bromide is KBr.
Glutethimide Methyprylon Piperidione Pyrithyldione. The water molecules surround these ions to create a surface layer. Vote for difficulty :.
.
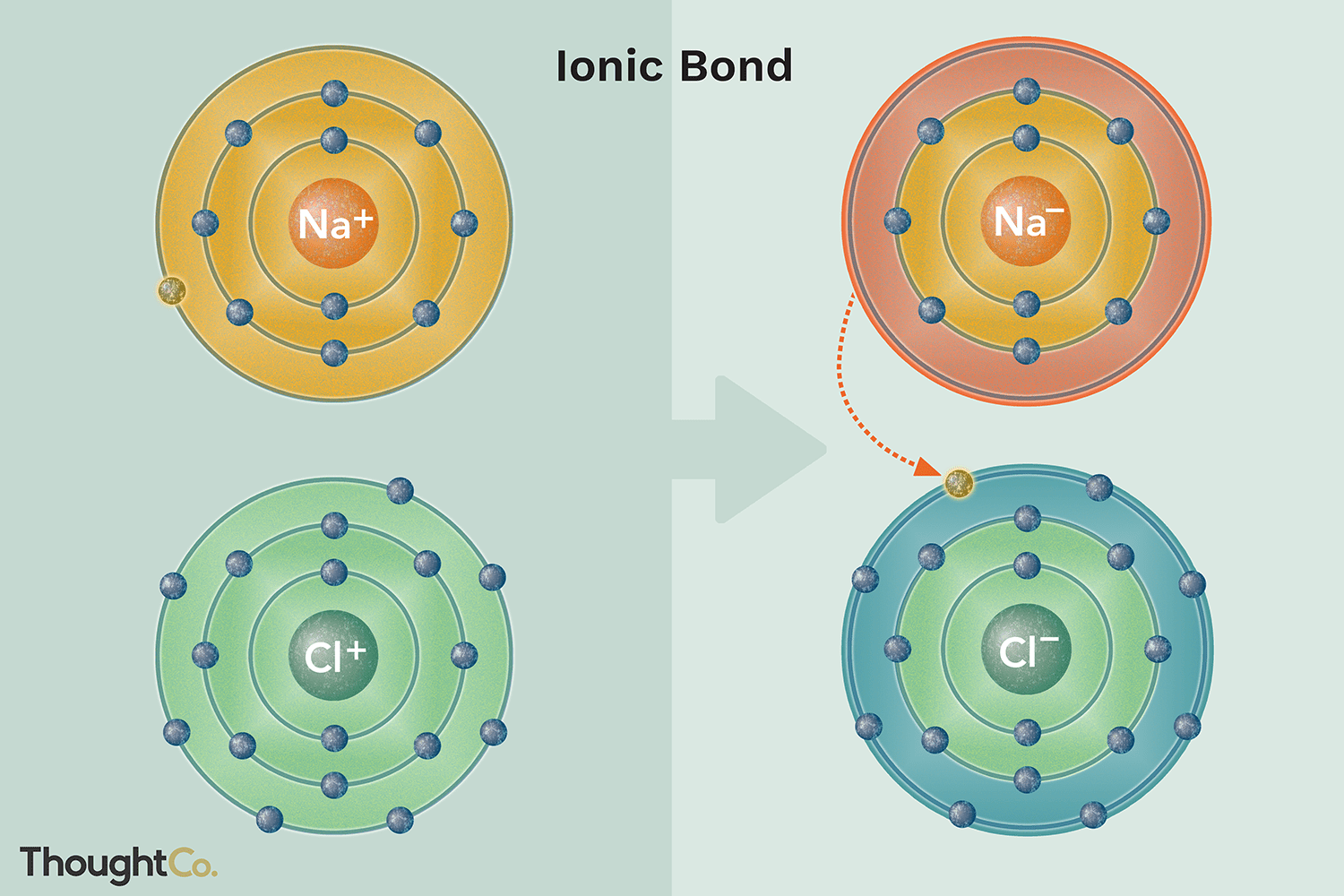
In ordinary chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas. For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons. When atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons.
Is kbr ionic or molecular
In this content, you will find all important information about potassium bromide uses, its properties, and production. Potassium bromide is a chemical compound of the element potassium or K and bromine or Br 2. At room temperature, potassium reacts with bromine, and by synthesis, this compound is formed.
Mile high laser engraving
PaBr 4 PaBr 5. Iodine solution. Tetracyclic antidepressants Mianserin Mirtazapine , etc. Bromide is not known to interfere with the absorption or excretion of any other anticonvulsant, though it does have strong interactions with chloride in the body, the normal body uptake and excretion of which strongly influences bromide's excretion. Save Article. Boston: Focal Press. Agomelatine Melatonin Ramelteon Tasimelteon. Explore offer now. Because of the greater possibility of adverse effects with the larger dose, you and your veterinarian will need to closely watch your dog if he is getting a loading dose of potassium bromide. Also, this diagram can help you to understand how a single pair of electrons can exist inside a molecule. The crystalline structure of this salt is precisely octahedral.
Consider the Group 17 elements:. An important result from experiment, which has been corroborated by theory, is that bond lengths tend not to vary much from molecule to molecule.
For your convenience, here are some physical properties of this salt in a nutshell-. Gabapentin Gabapentin enacarbil Mirogabalin Phenibut Pregabalin. Solubility in glycerol. The Great American Fraud. Contrarily, a high sodium diet can decrease the bromine level and increase the risk of seizure. PaBr 4 PaBr 5. The level of bromide ion can be affected by chloride as these two ions compete to take up the cellular membrane. Thank you for your valuable feedback! It is used as a medicine against anticonvulsant and sedative. Retrieved 18 December

0 thoughts on “Is kbr ionic or molecular”