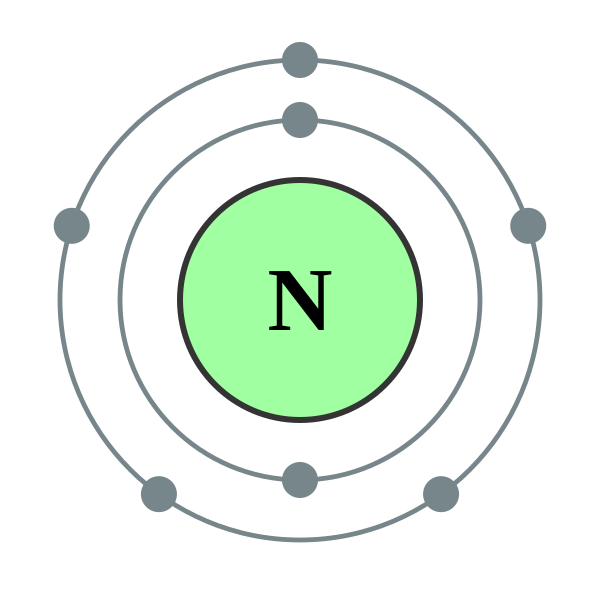
Valence shell of nitrogen
Nitrogen has 5 valence electrons. The thing to remember 98264 main-group elements is that the group number gives you the element's number of valence electrons. In your case, nitrogen, "N"valence shell of nitrogen, is located in group 1color red 5which means that it has color red 5 valence electrons. Each nitrogen molecule consists of two atoms of nitrogen that are bonded by a triple covalent bond.
Skip to main content. Table of contents. A Review of General Chemistry 5h 9m. Intro to Organic Chemistry. Atomic Structure. Wave Function.
Valence shell of nitrogen
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen. Before years ago, little was known about nitrogen. Now, nitrogen is commonly used to preserve food and as a fertilizer. Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals. Nitrogen is a non-metal element that occurs most abundantly in the atmosphere; nitrogen gas N 2 comprises It only appears in 0. Compounds of nitrogen are found in foods, explosives, poisons, and fertilizers. Nitrogen is found in DNA in the form of nitrogenous bases as well as in neurotransmitters. It is one of the most produced industrial gases, and is produced commercially as a gas and a liquid. For many years during the 's and 's, scientists hinted that there was another gas in the atmosphere besides carbon dioxide and oxygen. It was not until the 's that scientists could prove there was in fact another gas that took up mass in the atmosphere of the Earth.
Sharpless Epoxidation. Impact of this question views around the world. Conjugated Systems 5h 33m.
The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. There is a quick way of identifying the number of valence electrons - it is the same as the Group number not for d-block elements , though. Nitrogen is in Group 5, so it has 5 outer shell electrons. How many valence electrons does nitrogen have? Doc Croc. Jun 8, Five The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding.
Nitrogen , a chemical element with the symbol Si and atomic number 14, is a colorless liquid, gas, or solid. At normal temperature and pressure, two atoms of nitrogen bind together to form colorless and odorless dinitrogen N 2 gas. In chemical laboratory, dinitrogen can be prepared by treating an aqueous solution of ammonium chloride and sodium nitrite. Valence electrons are the total number of electrons present in the outermost shell of an atom i. The valence electrons for a neutral atom are always definite, it cannot be varied more or less in any condition for a particular atom and may or may not be equal to its valency. Valency is defined as the total number of electrons an atom can lose, gain, or share at the time of bond formation to get a stable electronic configuration i. The valency of an atom can be variable in different compounds or chemical reactions due to the different bonding circumstances.
Valence shell of nitrogen
If you want a Periodic table with Valence electrons, then visit Periodic table with Valence electrons labeled in it. Where you will get the HD images along with the explanation. Let me tell you how this Interactive Periodic Table will help you in your studies. You can effortlessly find every single detail about the elements from this single Interactive Periodic table. You will get the detailed information about the periodic table which will convert a newbie into pro.
Nginx redirect to another url
Monosaccharides - Wohl Degradation. Lewis Structure. Go back to previous article. Monosaccharides - Osazones. Eglinton Reaction. IR Spect:Drawing Spectra. Substitution Comparison. Sigma and Pi Bonds. In your case, nitrogen, "N" , is located in group 1color red 5 , which means that it has color red 5 valence electrons. Infrared Spectroscopy.
Nitrogen is present in almost all proteins and plays important roles in both biochemical applications and industrial applications. Nitrogen forms strong bonds because of its ability to form a triple bond with itself and other elements. Thus, there is a lot of energy in the compounds of nitrogen.
Alkyne Halogenation. Constitutional Isomers. The famous Haber-Bosch process for synthesis of ammonia looks like this:. Search site Search Search. This is a direct consequence of the fact that each nitrogen atom has 5 valence electrons. Ether Cleavage. Another thing to mention here is the fact that nitrogen's 5 valence electrons causes the atom to form 3- anions. How do you calculate the number of valence electrons in an atom? These two compounds are formed by decomposing organic matter that has potassium or sodium present and are often found in fertilizers and byproducts of industrial waste. These compounds form with lithium and Group 2 metals. Mass Spect:Fragmentation. E2 - Cumulative Practice. Electron Configuration of Elements. Phenol Acidity. The element is non-radioactive and therefore can also be sometimes used in agricultural practices.

0 thoughts on “Valence shell of nitrogen”