Phospholipase a2
Federal government websites often end in.
Inflammation and Regeneration volume 36 , Article number: 7 Cite this article. Metrics details. Within the phospholipase A 2 PLA 2 superfamily that hydrolyzes phospholipids to yield fatty acids and lysophospholipids, the secreted PLA 2 sPLA 2 enzymes comprise the largest family that contains 11 isoforms in mammals. Individual sPLA 2 s exhibit unique distributions and specific enzymatic properties, suggesting their distinct biological roles. While sPLA 2 s have long been implicated in inflammation and atherosclerosis, it has become evident that they are involved in diverse biological events through lipid mediator-dependent or mediator-independent processes in a given microenvironment. In recent years, new biological aspects of sPLA 2 s have been revealed using their transgenic and knockout mouse models in combination with mass spectrometric lipidomics to unveil their target substrates and products in vivo.
Phospholipase a2
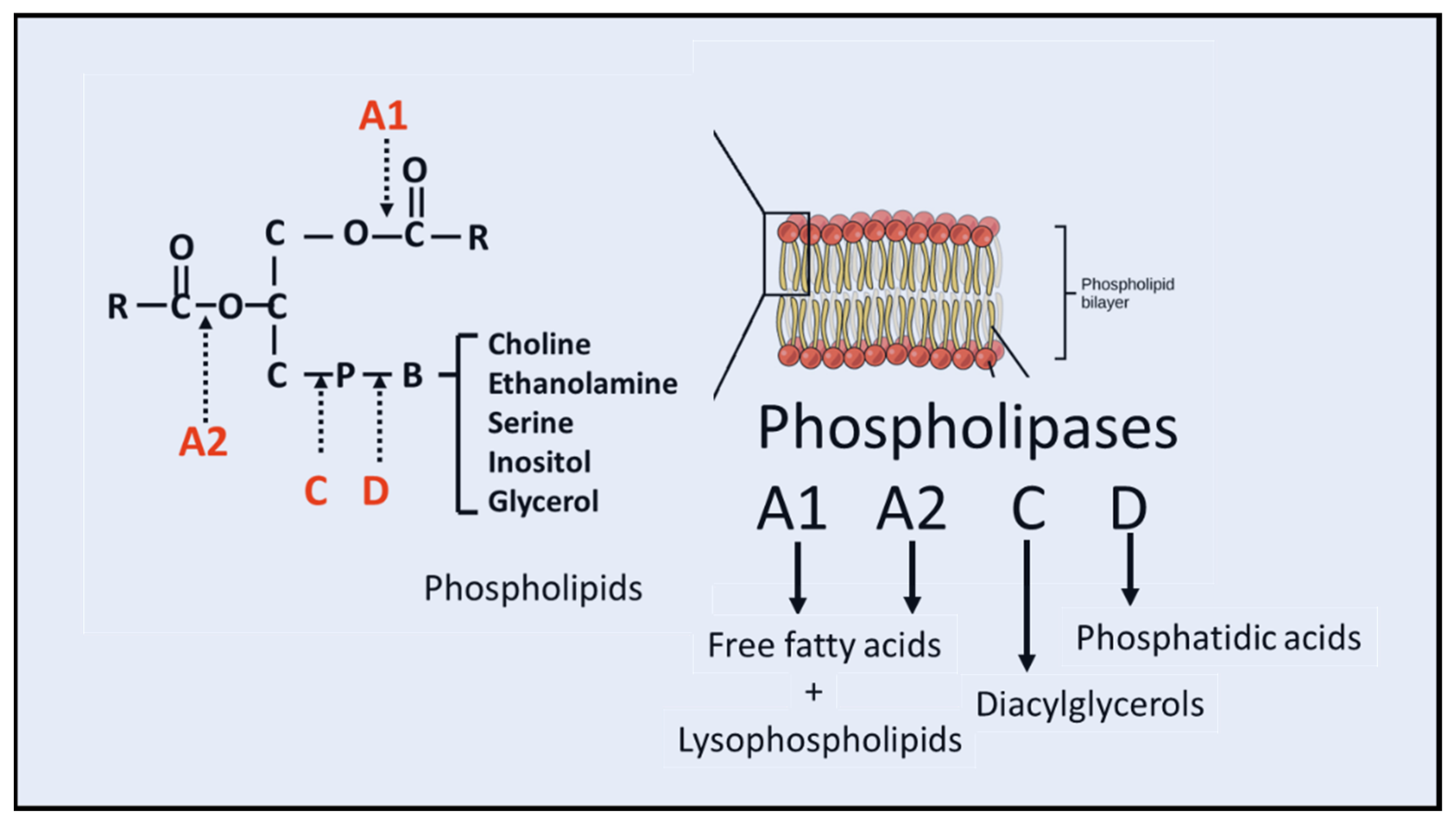
The enzyme phospholipase A 2 EC 3. This particular phospholipase specifically recognizes the sn 2 acyl bond of phospholipids and catalytically hydrolyzes the bond, releasing arachidonic acid and lysophosphatidic acid. Upon downstream modification by cyclooxygenases or lipoxygenases , arachidonic acid is modified into active compounds called eicosanoids. Eicosanoids include prostaglandins and leukotrienes , which are categorized as anti-inflammatory and inflammatory mediators. PLA2 enzymes are commonly found in mammalian tissues as well as arachnid, insect, and snake venom. Due to the increased presence and activity of PLA2 resulting from a snake or insect bite, arachidonic acid is released from the phospholipid membrane disproportionately. As a result, inflammation and pain occur at the site. Additional types of phospholipases include phospholipase A 1 , phospholipase B , phospholipase C , and phospholipase D. Phospholipases A 2 include several unrelated protein families with common enzymatic activity. Two most notable families are secreted and cytosolic phospholipases A 2.
Read Edit View history. Bayburt T, Gelb MH. Increased sPLA2 activity is observed in the cerebrospinal fluid of humans with Alzheimer's disease and multiple sclerosisand may serve as a marker of increases in phospholipase a2 of the blood-cerebrospinal fluid barrier.
.
Federal government websites often end in. The site is secure. The data presented in this review were compiled from the cited work. Details about the adapted figures and graphic are available upon request. The phospholipase A2 PLA2 superfamily of phospholipase enzymes hydrolyzes the ester bond at the sn-2 position of the phospholipids, generating a free fatty acid and a lysophospholipid. The superfamily of PLA2 comprises at least six big families of isoenzymes, based on their structure, location, substrate specificity and physiologic roles.
Phospholipase a2
Federal government websites often end in. Before sharing sensitive information, make sure you're on a federal government site. The site is secure. NCBI Bookshelf. Jarett Casale ; Salah Eddine O. Kacimi ; Matthew Varacallo. Kacimi 2 ; Matthew Varacallo 3. Phospholipase A PLA comprises a supergroup of esterase enzymes present in all human cells that play a key role in mediating the production of free fatty acids and lysophospholipids from glycerophospholipids. These enzymes are essential for regulating homeostasis and disease pathogenesis in every organ system based on their activation and involvement in inflammatory mediation. Over 30 isoforms of PLA have been identified to date and differ heavily in function, cofactor requirement, and size.
Taylor marie hill
Nat Immunol. Enhanced activity and altered specificity of phospholipase A2 by deletion of a surface loop. Biochim Biophys Acta. Biochemical characterization of various catalytic complexes of the brain platelet-activating factor acetylhydrolase. J Biol Chem. In general, this reaction is best known as the initial, rate-limiting step of arachidonate metabolism leading to the production of bioactive lipid mediators including prostaglandins and leukotrienes. Systemic lipid metabolism is often affected by the digestion and absorption of dietary lipids in the GI tract. Bee venom phospholipase A 2 sPLA2. Role of group II secretory phospholipase A 2 in atherosclerosis: 1. The first cytosolic PLA 2 was isolated from neutrophils and platetlets in Expression of group IA phospholipase A 2 in Pichia pastoris : identification of a phosphatidylcholine activator site using site-directed mutagenesis. Full size image. The molecular basis of differential subcellular localization of C2 domains of protein kinase C-alpha and group IVa cytosolic phospholipase A2. J Cell Sci.
The enzyme phospholipase A 2 EC 3.
Background Phospholipase A 2 PLA 2 is a group of enzymes that hydrolyze phospholipids to yield fatty acids and lysophospholipids Fig. This enzyme has also been shown to be activated by phospholipids containing phosphatidylcholine PC head groups [ 56 ], and two possible sites for this interaction have been suggested [ 41 , 57 ]. They release arachidonic acid from membrane phospholipids. Kinetic properties of a high molecular mass arachidonoyl-hydrolyzing phospholipase A2 that exhibits lysophospholipase activity. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. Cloning of a gene for a novel epithelium-specific cytosolic phospholipase A2, cPLA2delta, induced in psoriatic skin. Together, these results reveal a functional link between lipoprotein metabolism and anti-inflammation for this particular sPLA 2 and provide a rationale for the long-standing issue of the physiological importance of lipoprotein hydrolysis by this extracellular enzyme family Fig. Burke 1, 2 and Edward A. A novel anti-inflammatory role for secretory phospholipase A 2 in immune complex-mediated arthritis. Conformation of micellar phospholipid bound to the active site of phospholipase A2. Probing the role of histidine, aspartic acid, cysteine, and arginine. Bibcode : PLoSO The active site residues Ser, Asp and Arg are shown in stick form colored red.

It is a shame!