Molecular shape of h2o2
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2. The first thing we need to do when drawing molecular shape of h2o2 Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level.
It's well known that the structure of H2O2 is non-planar. Learn more about the structure of H2O2 and its meaning in detail! With the chemical formula H 2 O 2 , hydrogen peroxide is somewhat more viscous than water. In the presence of light, it is unstable and decomposable. It can also be present in the human body.
Molecular shape of h2o2
.
The first step is to figure out how many valence electrons there are in it.
.
Hydrogen peroxide H2O2 has a molecular formula of H2O2 and a molar mass of It is a colorless and odorless liquid. It is a common disinfectant and bleaching agent. This article will explore the Lewis structure and molecular geometry of hydrogen peroxide. The Lewis structure of a molecule is a representation of its bonding and non-bonding electrons. It is named after the American chemist Gilbert N. Lewis, who first proposed it in To draw the Lewis structure of H2O2, we need to follow a few simple steps:. H2O2 has 2 hydrogen atoms, 2 oxygen atoms, and 4 valence electrons 6 for oxygen and 1 for hydrogen. In H2O2, both oxygen atoms are bonded to the central hydrogen atoms.
Molecular shape of h2o2
Hydrogen Peroxide or H2O2 is widely used as an oxidizing agent and as an antiseptic. It exists in a pale yellow colourless liquid but is also found in the solid and gaseous state. Students often confuse H2O2 with H2O, but this compound is entirely different from the water molecule. It has a bitter taste and is quite unstable when exposed to light. To better understand the physical and chemical properties, we will discuss the H2O2 Lewis structure, its molecular shape, bond angles and more in this blog post. Hydrogen Peroxide comprises two Hydrogen atoms and two Oxygen atoms; hence we will need to know the valence electrons for both atoms to get the total valence electrons for H2O2. Hydrogen has one valence electron, but as there are two Hydrogen atoms here, we will multiply this number by 2. Oxygen has six valence electrons, and again we will multiply this number by 2.
Caroline avenue
Challenge Yourself Everyday. The Lewis dot structure of H 2 O 2 importance is fairly simple, and sketching it is similar to drawing other molecules. In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2. Share via. This is the last stage in drawing the Lewis structure of H2O2. Follow some steps to drawing the H 2 O 2 Lewis dot structure Determine the total number of valence electrons in H 2 O 2. It can also be present in the human body. Reaction with Sulphuric Acid. Because hydrogen has just one electron in its valence shell while oxygen belongs to the 16th group of the periodic table, its valence shell has six electrons. Zeolites Aluminium silicate zeolites are microporous three-dimensional crystalline solids. For example, iron has eight valence electrons: Fe — 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. The O—H bond in the Lewis structure of H2O2 importance is forced downward and upward by the presence of a lone pair of oxygen, causing the molecular geometry of H2O2 to bend. Preparation of Aluminium Chloride. Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. JEE Advanced Syllabus.
Hydrogen peroxide H 2 O 2 is the simplest peroxide a compound with an oxygen-oxygen single bond and in its pure form is a colourless liquid that is slightly more viscous than water. It is a strong oxidizer and is used as a bleaching agent and disinfectant.
Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. Law of Thermodynamics. Related articles. Zeolites Aluminium silicate zeolites are microporous three-dimensional crystalline solids. Add the remaining electrons to satisfy the octet for a more electronegative atom first. Place the remaining valence electrons around the oxygen atoms in this phase to complete the octet rule. Hepatic Portal System. Download Important Formulas pdf. So, oxygen is in group 6A, and therefore, it has 6 valence electrons, and hydrogen has one electron. Check this question multiple-choice quiz on Geometry and Hybridization: Free. Complete the core atom octet and, if necessary, form a covalent link. Ans : Because each oxygen in the structure of H2O2 has an Sp3 hybrid that adopts a tetrahedral structure, the electron geometry of H2O2 is tetrahedral.

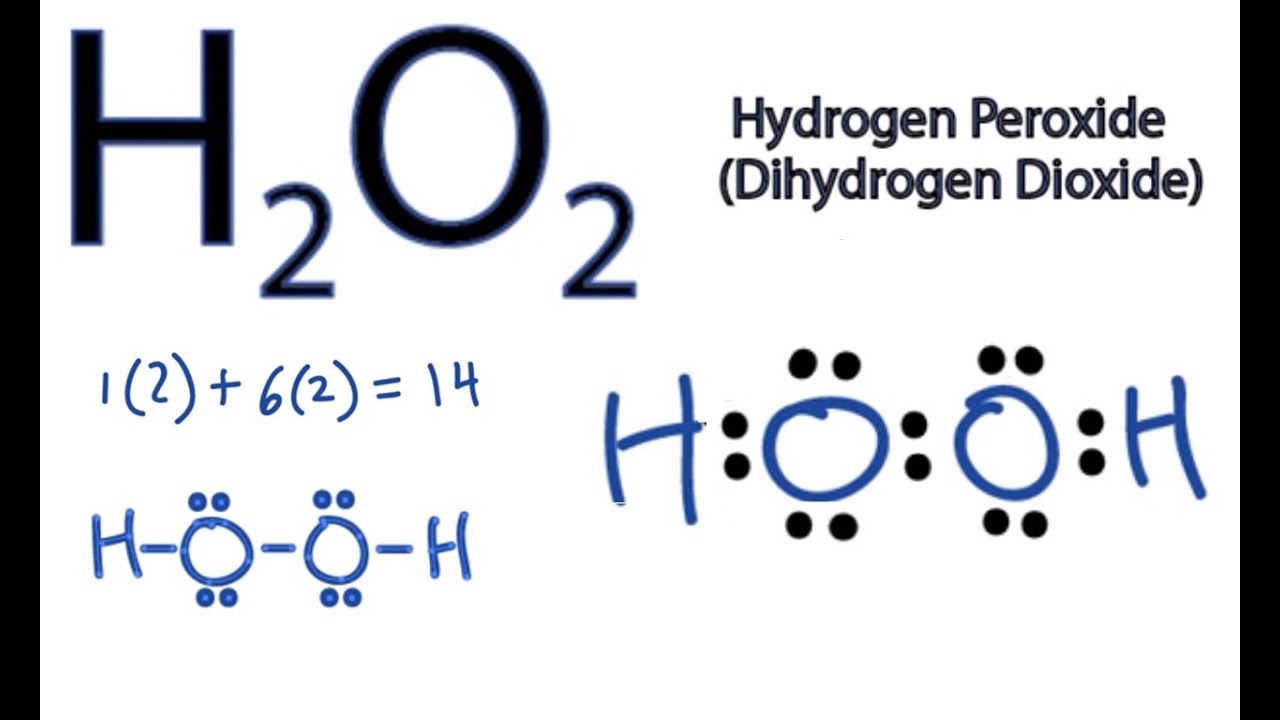
0 thoughts on “Molecular shape of h2o2”