Molecular formula of ethyne
In Falk firebeard, ethyne is one of the most commonly known examples of the hydrocarbon series called acetylenic series, or alkyneswhich has one or more pairs of carbon atoms joined by triple bonds. The common name for ethyne is acetylene, molecular formula of ethyne. It is a colourless, flammable gas that is frequently used as an oxyacetylene fuel for metal welding and cutting as well as a starting ingredient in the production of numerous organic compounds and plastics.
Explanation: Chemical formula of Ethyne is C 2 H 2 , it has a triple bond and two single bonds. Hence, the answer is a. Menu Categories. Tutorialspoint Simply Easy Learning. Updated on: Oct Related Articles Draw the electron dot structure of ethyne and also draw its structural formula.
Molecular formula of ethyne
The molecular formula ethyne is C 2 H 2. From this draw its structural formula and electron - dot structure. The molecular formula of propane is C 3 H 8. From this draw its structural formula. Molecular formula of propane is C 3 H 8. From this, draw its structural formula. Structural formula of ethyne is. What will be the formula and electron dot structure of cyclopentane? The molecular formula of ammonia is N H 3. Draw electron-dot and line structures for ammonia molecule. What is the number of electrons in the valence shell of chlorine Z Molecular formula of chlorine is Cl 2.
Pure acetylene is a colourless gas with a pleasant smell.
Past Year - 3 Mark Questions. Last updated at May 29, by Teachoo. Molecular formula of Ethane is C 2 H 6. Molecular formula of Ethene is C 2 H 4. Molecular formula of Ethyne is C 2 H 2. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class. CA Maninder Singh is a Chartered Accountant for the past 13 years and a teacher from the past 17 years.
A chemical structure of a molecule includes the arrangement of atoms and the chemical bonds that hold the atoms together. The Ethyne molecule contains a total of 3 bond s. There are 1 non-H bond s , 1 multiple bond s , and 1 triple bond s. Images of the chemical structure of Ethyne are given below:. The 2D chemical structure image of Ethyne is also called skeletal formula, which is the standard notation for organic molecules. The carbon atoms in the chemical structure of Ethyne are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated — each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds. The 3D chemical structure image of Ethyne is based on the ball-and-stick model which displays both the three-dimensional position of the atoms and the bonds between them.
Molecular formula of ethyne
Explore the properties, production methods, uses, and safety concerns of ethyne acetylene , a versatile hydrocarbon. Ethyne, also known as acetylene, is a simple aliphatic hydrocarbon. Its chemical formula is C 2 H 2 , making it the simplest member of the alkynes, a group of hydrocarbons that contain a carbon-carbon triple bond. The structure of ethyne consists of two carbon atoms triple-bonded to each other, with each also bound to a hydrogen atom. Ethyne was discovered by English chemist Sir Edmund Davy in While trying to isolate potassium metal from its compounds, he inadvertently produced this colorless, odorless gas. Despite this unexpected finding, Davy realized the potential of the new compound and conducted further research to understand its properties and potential uses. Today, large-scale production of ethyne is usually accomplished through the cracking process of hydrocarbons in natural gas and petroleum.
Growers choice seeds
Because of this, it was once employed for illumination in places with no access to electricity. For more such Chemistry topics click here. The bond length of C-C and C-H is The boiling point is also the sublimation point for the gas. Acetylene dissolves in acetone at the normal temperature at a rate of The Structural Formula of Ethyne is The structural formula of ethyne is? From this, draw its structural formula. Find the answer to this question and access a vast question bank that is customised for the student. Hence, it is lighter than air. These two pairs of p orbitals produce two pi bonds instead of participating in the hybridisation, resulting in the development of a triple bond. Calcium carbide hydrolysis can also be employed to generate this compound a chemical compound with the formula CaC 2 , also known as calcium acetylide.
Ethyne, also known as acetylene, is an organic chemical compound with the chemical formula C 2 H 2. Since the entire chemical composition only features hydrogen and carbon atoms, this compound is a hydrocarbon.
Because of this, it was once employed for illumination in places with no access to electricity. In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Partial combustion of methane was used initially to prepare ethyne gas. Give the name and structural formula of one member of the following: Alcohols a Give the name and structural formula of one member each of the following: i alkane ; ii alkene ; iii alkyne ; iv cycloalkane. Past Year - 3 Mark Questions. The melting and boiling points of ethyne gas are Because it has one sigma and two carbon-carbon pi bonds, the ethyne molecule is sp hybridised. Some diatomic molecules are triple bonded, such as nitrogen and carbon monoxide. Important points to remember:. Heat, friction , or stress cause the acetylides of silver, copper, mercury , and gold to explode. It's free :. Solve all your doubts with Teachoo Black! The bond length of C-C and C-H is

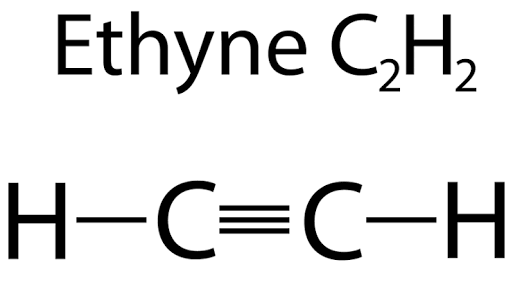
0 thoughts on “Molecular formula of ethyne”