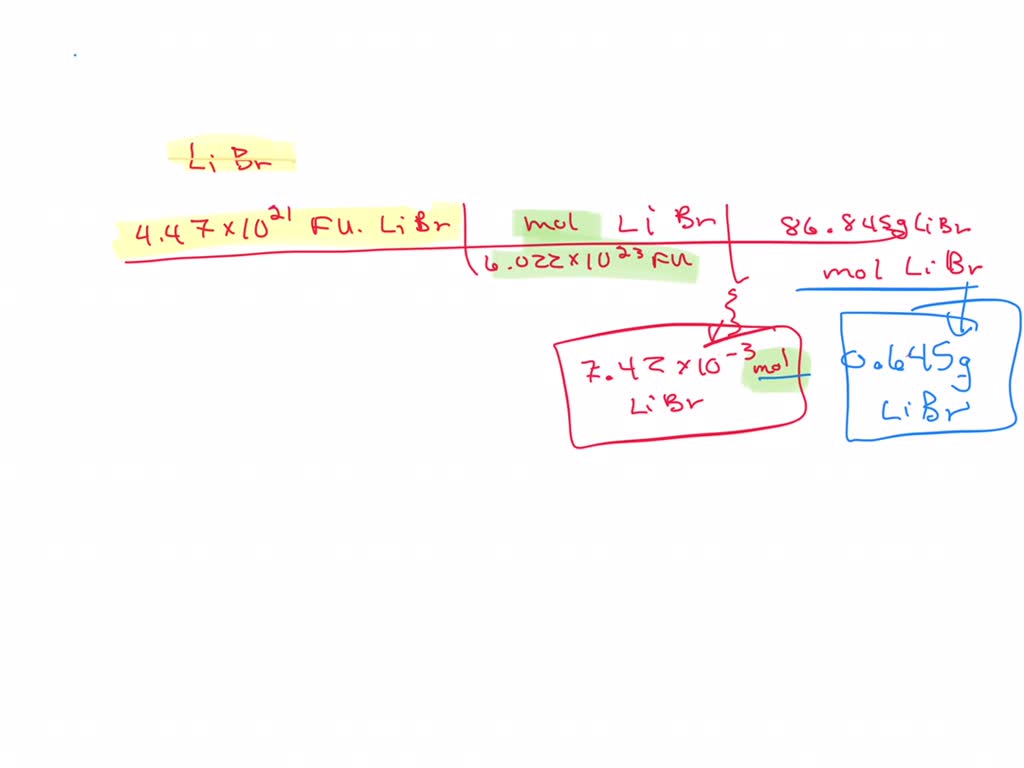
Molar mass libr
Then, lookup atomic weights for each element in periodic table : Li: 6.
Did you know that some animals are much better at differentiating colors than humans are and they can even see the ultraviolet and the infrared light? Click or tap to find more info about wavelength and color! All substances consist of atoms or molecules. In chemistry, it is important to measure their amounts accurately. The mole is used to express the amounts of reactants and products of chemical reactions.
Molar mass libr
Random converter. So, you like coffee? Find out what pressure is necessary to make really good espresso! All substances consist of atoms or molecules. In chemistry, it is important to measure their amounts accurately. The mole is used to express the amounts of reactants and products of chemical reactions. The mole, symbol mol, is the SI unit of the amount of substance. One mole contains exactly 6. The amount of substance, symbol n , of a system is a measure of the number of specified elementary entities. An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles. In other words, the mole is the amount of substance equal in mass to the combined mass in atomic mass units of the atoms of molecules of the substance multiplied by the Avogadro constant or Avogadro number. The mole as the unit of measurement for the amount of substance is one of the seven base units of the International System of Units SI. Its symbol is mol. One mole of pure carbon has a mass of exactly 12 grams.
Agomelatine Melatonin Ramelteon Tasimelteon. Then, lookup atomic weights for each element in periodic table : Li: 6.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. A common request on this site is to convert grams to moles.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Molar mass libr
Molar mass of LiBr Lithium bromide is Then, lookup atomic weights for each element in periodic table : Li: 6. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in LiBr: Li: 1, Br: 1 Then, lookup atomic weights for each element in periodic table : Li: 6. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element.
Colossus movie theatre langley bc
Other anions. GHS labelling :. FeBr 2 FeBr 3. Salts and covalent derivatives of the bromide ion. Infobox references. Other cations. Vi garanterer dog ikke, at vores omformere og regnemaskiner er fri for fejl. Mole is a standard scientific unit for measuring large quantities of very small entities such as atoms and molecules. Y verify what is Y N? Oxygen O has an atomic mass of about Molar mass of LiBr, Lithium Bromide is Chemical forum. Lethal dose or concentration LD, LC :.
Molar mass of LiBr Lithium bromide is
FREE Signup. Common compound names. Using the Molar Mass Calculator Converter This online unit converter allows quick and accurate conversion between many units of measure, from one system to another. Chemical forum. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. Terms and Conditions. Tools Tools. Do you have difficulty translating a measurement unit into another language? An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles. To complete this calculation, you have to know what substance you are trying to convert. Burning is a high-temperature exothermic redox chemical reaction. Classification Of Carbohydrates. GeBr 2 GeBr 4.

0 thoughts on “Molar mass libr”