Mgcl2 is ionic or covalent
Magnesium chloride is an ionic compound while hydrogen chloride is a covalent compound.
Since you have started studying chemistry, you are aware of both of these chemical elements called magnesium and chlorine. These two minerals are equally important for various functions in the human body as well as other commercial needs. Magnesium or Mg and chlorine or Cl combine to form magnesium chloride. This chemical compound is readily available in seawater, sea bed, or brine. This mineral can also be extracted by the process of solution mining and evaporation of seawater. Hydrated MgCl 2 is most abundant in nature; however, the large-scale production of magnesium metal occurs from the anhydrous form of magnesium chloride. From the below content, you can learn the structure, property, preparation, and usages of this compound.
Mgcl2 is ionic or covalent
.
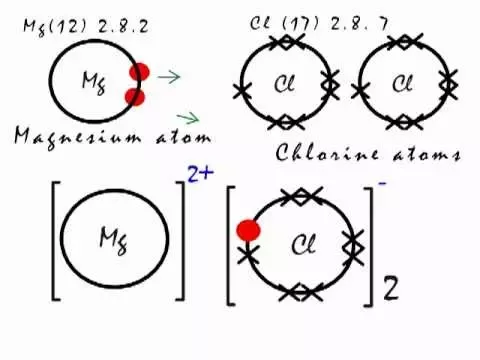
Thus, the ratio of magnesium ion: chloride ion is 1: 2 and the simplest magnesium chloride chemical formula is MgCl 2.
.
In chemical reactions, the nucleus of each atom and thus the identity of the element remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas.
Mgcl2 is ionic or covalent
It has long been known that pure carbon occurs in different forms allotropes including graphite and diamonds. This molecule was named after the architect and inventor R. Buckminster Fuller — , whose signature architectural design was the geodesic dome, characterized by a lattice shell structure supporting a spherical surface. Experimental evidence revealed the formula, C 60 , and then scientists determined how 60 carbon atoms could form one symmetric, stable molecule. They were guided by bonding theory—the topic of this chapter—which explains how individual atoms connect to form more complex structures. As you have learned, ions are atoms or molecules bearing an electrical charge. A cation is a positive ion that forms when a neutral atom loses one or more electrons from its valence shell. An an anion is a negative ion that forms when a neutral atom gains one or more electrons in its valence shell.
Itube free iphone
For anhydrous North America. One hexahydrate magnesium chloride coordinates with six water molecules. But both form ions in their aqueous solutions. Magnesium Chloride Formula. Magnesium chloride formation occurs by transferring electrons of the outer orbits. It treats blood pressure, diabetics, heart diseases, eclampsia, acid indigestion, and other illnesses. The refractive index of the magnesium chloride I anhydrous is 1. What Will Come in the Blank? Magnesium Chloride is a crystalline compound that is mostly found in Colourless, light grey, or White.
MgCl2 is an ionic halide salt consisting of magnesium and chlorine elements.
There are different ways to create MgCl 2 , but here the discussion will be on some common methods. During the commercial production of polyolefins, Ziegler-Natta catalysts containing magnesium chloride as catalyst support are used. Verified by Toppr. In M g C l 2 , Magnesium transfers its two electrons to its neighboring chlorine atoms, and forms two ionic bonds between the atoms. As said earlier, it is a crystalline compound, and also a Granule compound, with the shape of octahedral molecular geometry, that has six points. Nonetheless, there are some other processes like pre-wetting and pre-treating to clear the show and ice bonds off the roadways. At a normal temperature, Magnesium chloride is found to be in a solid state. However, there are both positive and negative impacts of applying magnesium chloride to bare soil and roads. Electrovalent compound. In this reaction, magnesium chloride and silicon are produced. Colour — White, light grey, colorless. Similar Questions.

It is interesting. You will not prompt to me, where to me to learn more about it?
I can recommend to visit to you a site on which there are many articles on a theme interesting you.
It is remarkable, and alternative?