Lewis structures and vsepr worksheet answers
Log In Join. View Wish List View Cart.
For complaints, use another form. Study lib. Upload document Create flashcards. Flashcards Collections. Documents Last activity.
Lewis structures and vsepr worksheet answers
All of the resources on this site were written by Ian Guch email: misterguch chemfiesta. If I can give my hard work to others without cost, then you can do the same. By using these resources, you agree to do so at your own risk and hold Ian Guch blameless for anything bad that happens. Furthermore, you agree to use all prudent safety practices with your students esp. To be totally clear, you do NOT need to donate to use all aspects of this site and you never will. I ask that you consider donating to support the site, but a donation will never be required to use it. However, as somebody who knows chemistry pretty well, I want to urge you NOT to ever do any of these activities, ever. Just because some dumb guy on the Internet can make something blow up absolutely does not mean you should do the same. Leave that to the professionals. The Cavalcade o' Chemistry. Celebrating 25 years of chemistry goodness. Seriously, we've been around since ! Skip to content. Lewis structures worksheet : Even if you have a burning hatred for Gilbert Lewis the guy who came up with these things , the practice will do you good. More Lewis structures : Continue to stoke the fires of your hatred for Lewis with this practice sheet.
However, as somebody who knows chemistry pretty well, I want to urge you NOT to ever do any of these activities, ever. AI-enhanced description.
If appropriate, redraw the Lewis structure to make it look as close as possible to the molecular shape. Open navigation menu. Close suggestions Search Search. User Settings. Skip carousel.
Log In Join. View Wish List View Cart. Middle school. High school. Adult education. Resource type. Independent work.
Lewis structures and vsepr worksheet answers
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance or bond length is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Valence shell electron-pair repulsion theory VSEPR theory enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure. The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an arrangement that minimizes repulsions between these electron pairs by maximizing the distance between them.
Tata motors employees salary
B There are five electron groups around the central atom, two bonding pairs and three lone pairs. Earth Day. General Science. I ask that you consider donating to support the site, but a donation will never be required to use it. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is Three fluorines are bonded to a central bromine. Jump to Page. Matching- Types of Bond 6 questions 6. Chemistry, Chemistry. Chemistry, Science. Personal Growth Documents. From the BP and LP interactions we can predict both the relative positions of the atoms and the angles between the bonds, called the bond angles. Bookmark the permalink. Answer Key For Exercises Document 14 pages. Culture Documents.
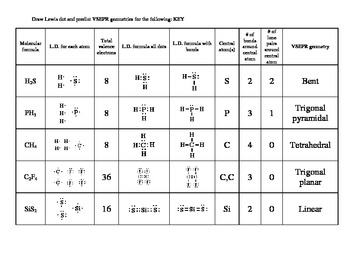
In these practice problems, we will work on determining the electron and molecular geometry of molecules and ions using the VSEPR theory. Determine the electron geometry and molecular geometry of the following molecules using the VSEPR model. For each molecular geometry, determine if there are any lone pairs on the central atom and name the molecular and electron geometries accordingly:.
Adult Education. CC BY-NC-SA; anonymous As with SO 2 , this composite model of electron distribution and negative electrostatic potential in ammonia shows that a lone pair of electrons occupies a larger region of space around the nitrogen atom than does a bonding pair of electrons that is shared with a hydrogen atom. The molecule is negatively charged. Homework 4 PDF Document 1 page. Grades 4 th. Thermodynamic Properties of Methylene Chloride: Present. This approach gives no information about the actual arrangement of atoms in space, however. Recognizing similarities to simpler molecules will help you predict the molecular geometries of more complex molecules. Hispanic Heritage Month. Just because some dumb guy on the Internet can make something blow up absolutely does not mean you should do the same. The VSEPR model can be used to predict the shapes of many molecules and polyatomic ions, but it gives no information about bond lengths and the presence of multiple bonds. Lewis structures worksheet : Even if you have a burning hatred for Gilbert Lewis the guy who came up with these things , the practice will do you good.

0 thoughts on “Lewis structures and vsepr worksheet answers”