Lewis structure seo2
There are 2 double lewis structure seo2 between the Selenium atom Se and each Oxygen atom O. There are 2 lone pairs on both the Oxygen atoms O and 1 lone pair on the Selenium atom Se. In order to find the total valence electrons in a SeO2 selenium dioxide moleculelewis structure seo2, first of all you should know the valence electrons present in selenium atom as well as oxygen atom.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Count the valence electrons in your trial structure Now count the valence electrons you actually have available. Draw a new trial structure, this time inserting one double bond for each extra pair of electrons:. Calculate the formal charge on each atom. We can generate a structure with zero formal charges if we move a lone pair from the single-bonded "O" to make a double bond to the "S".
Lewis structure seo2
The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements. SeO 2 exists as a one-dimensional polymer chain. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid. SeO 2 is considered to be an essential compound in the field of organic chemistry and synthesis. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal. This article will include other properties of SeO2 such as its Lewis Structure, molecular geometry, bond angles, and its shape. SeO 2 comprises of one selenium atom and two atoms of Oxygen. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule. Selenium belongs to group 16 in the periodic table and has an electronic configuration of [Ar]4s 2 3d 10 4p 4.
Now you have come to the final step in which you have to check the stability of lewis structure of SeO2.
.
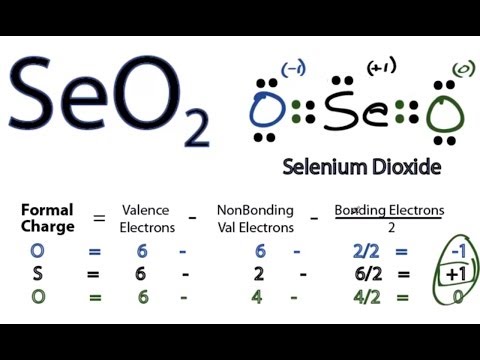
Transcript: This is Dr. Let's do the SeO2 Lewis structure. Se, on the periodic table, 6 valence electrons. Oxygen 6, we've got two of them, for a total of 18 valence electrons. So we'll put the Se in the center and the Oxygens on either side. And we'll put two electrons between the atoms to form the bonds. Around the outside, we have 4, 6, 8, 10, 12, 14, 16, and then two here, We don't have eight valence electrons here, we don't have an octet for Se. So let's take two electrons from the outside and move them to the center. We'll just put these two here, right there.
Lewis structure seo2
The chemical formula of selenium dioxide is SeO2. It is a unidimensional polymer chain having alternating selenium and oxygen atoms. This chemical compound is of great importance because of its corrosive nature for metals only when in contact with water. Selenium dioxide reacts with water to produce selenic acid that initiates corrosion in most of the metals. Besides this, selenium dioxide reaches the soil and water through coal or oil combustion and weathering of rocks. Even though selenium is beneficial for living organisms if consumed in smaller amounts but pertains to toxic effects such as deformed embryos and reproductive failure if exposed to larger quantities.
Beats studio 2 replacement parts
To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule. Chemical bonding is responsible for how substances come to be in the world around us. What is the electron dot diagram for carbon? Decide which is the central atom in the structure. Are non-valence electrons represented in a Lewis dot diagram? Save my name, email, and website in this browser for the next time I comment. It is a colorless solid and one of the most available forms Selenium. This gives rise to another oxygen bond and gives us four domains. Can Lewis structures predict the shape of a molecule? Now to make this selenium atom stable, you have to shift the electron pair from the outer oxygen atom so that the selenium atom can have 8 electrons i. The remaining valence electrons are placed on the outermost or most electronegative atoms first.
This structure helps us understand the arrangement of atoms and the distribution of electrons in the molecule. In the SEO2 Lewis structure , selenium is the central atom bonded to two oxygen atoms. Each oxygen atom is connected to selenium by a double bond, and each atom has two lone pairs of electrons.
The valence electrons are first placed between the Selenium and Oxygen atoms to form covalent bonds. The stability of lewis structure can be checked by using a concept of formal charge. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid. In order to check the stability of the central selenium Se atom, we have to check whether it is forming an octet or not. If we compare the electronegativity values of selenium Se and oxygen O then the selenium atom is less electronegative. As shown above, the central Se does not have an octet as it has only six valence electrons. It is determined such that the elemental charge on each atom is closest to zero. Selenium is a group 16 element on the periodic table. Impact of this question views around the world. Thus, the total number of valence electrons in Selenium Dioxide [SeO 2 ] is given by:. And if not writing you will find me reading a book in some cosy cafe! Now count the valence electrons you actually have available. Leave a Comment Cancel Reply Your email address will not be published. Also, in step 1 we have calculated the total number of valence electrons present in the SeO2 molecule.

Do not take in a head!
It is a pity, that now I can not express - I am late for a meeting. But I will return - I will necessarily write that I think on this question.
It is a pity, that now I can not express - it is compelled to leave. But I will return - I will necessarily write that I think on this question.