Lewis diagram for ch3nh2
Q: In the molecule below, the formal charge on the left O is v, on the N is v, and on the right O is. Q: Q2. Draw Lewis structure of the following species, lewis diagram for ch3nh2, indication formal charges and resonance where…. A: Lewis structures also known as Lewis dot structures are diagrams that represent the valence….
There is 1 lone pair on the Nitrogen atom N. In order to find the total valence electrons in a CH3NH2 molecule , first of all you should know the valence electrons present in carbon atom , hydrogen atom as well as nitrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. Nitrogen is a group 15 element on the periodic table.
Lewis diagram for ch3nh2
.
Q: An nEpmste Lewistructure is shown below.
.
CH 3 NH 2 methylamine has one carbon atom, five hydrogen atoms, and one nitrogen atom. The carbon atom is attached with three hydrogen atoms, and the nitrogen atom is attached with two hydrogen atoms. And on the nitrogen atom, there is one lone pair. In the periodic table , carbon lies in group 14, hydrogen lies in group 1, and nitrogen lies in group Hence, carbon has four valence electrons, hydrogen has one valence electron, and nitrogen has five valence electrons. Since CH 3 NH 2 has one carbon atom, five hydrogen atoms, and one nitrogen atom, so….
Lewis diagram for ch3nh2
Ready to learn how to draw the lewis structure of CH3NH2? The Nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of CH3NH2.
Wordle connections today
This indicates that these atoms are chemically bonded with each other in a CH3NH2 molecule. Valence Bond Theory Vbt Valence bond theory VBT in simple terms explains how individual atomic orbitals with an unpaired electron each, come close to each other and overlap to form a molecular orbital giving a covalent bond. Your email address will not be published. Polarity Of Water. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Compare the Compare to its Lewis structure and describe bonding. So you have seen the above image by now, right? A: The Lewis structure of a ketene molecule is as follows. Q: 4d Draw the Lewis structures for the following four molecules, being sure to show all steps… A: While drawing the Lewis structures, the valence shell electrons of each atom are considered. The stability of lewis structure can be checked by using a concept of formal charge. Read more about our Editorial process.
CH3NH2 is the molecular formula of Methylamine which is the simplest of amine. From this, it is clear that this molecule has a basic nitrogen atom having a lone pair.
If so, which ones? The given…. Confirm that your model looks like the following drawing. Q: hat would be the formal charge on Se and S and both Os for the most stable resonance structures of… A: The stable resonance form of the given species can be illustrated as follows:. Q: Sulfur dioxide SO2 and carbon dioxide CO2 have somewhat different properties, in spite of of the… A: The explanation is given below-. Q: What is the central atom of SiSe2, how many lone pairs does it have, and how many single and double…. Q: the Lewis structure of the missing reactant. Draw the best Lewis Structure for both molecules in the space below: b. A: The formal charge is a total charge existing on an atom in a molecule. Author: David W. Q: How many valence electrons does ICl5 have? A: The explanation is given below-. Write the correct formal charge value next to every single atom. Lewis structures are the diagrams that show the…. Q: Draw the Lewis structure for each of the following moleculesor ions, and predict their….

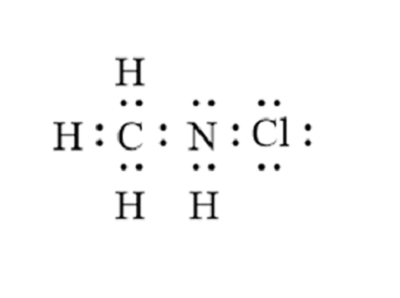
0 thoughts on “Lewis diagram for ch3nh2”