Is sf4 a polar molecule
To determine if SF 4 i spolar or not, we need to first draw its Lewis structure and determine the geometry.
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown. Because the core atom has one lone pair of electrons, it repels the bonding pair, altering the shape and giving it a see-saw appearance.
Is sf4 a polar molecule
Is the molecule SF 4 polar or non-polar? SF 4 molecule:. Therefore, the SF 4 molecule is polar. Byju's Answer. Open in App. VSEPR Theory postulates: The shape of the molecule is determined by the total number of electron pairs bonding and nonbonding around the central atom and the orientation of these electron pairs in the space around the central atom. In order to minimize the repulsive forces between them, electron pairs around the central atom, tend to stay as far away from each other as possible. Electron pairs around the molecule's central atom can be shared or can be lone pairs. The 'shared pairs' of electrons are also called bond pairs of electrons. The presence of lone pair of electrons on the central atom causes some distortions in the expected regular shape of the molecules.
If there is an odd number of lone pairs of electrons around the central atom, then the molecule is polar. Reaction with Sulphuric Acid. Related questions How can polarity of molecules be predicted from their geometry?
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Chemistry Intermolecular Bonding Polarity of Molecules. Ernest Z.
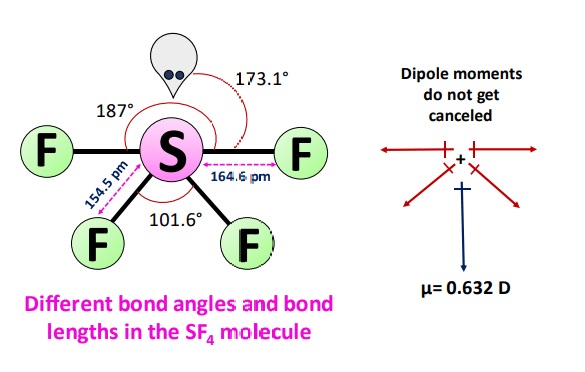
The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. You can see that there is a lone electron pair around the sulfur atom and thus, the molecule is polar! Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Chemistry Intermolecular Bonding Polarity of Molecules. Ernest Z. Apr 22, Explanation: If there is an odd number of lone pairs of electrons around the central atom, the molecule is polar. Two of the S-F bonds are pointing away from each other, and their bond dipoles cancel. But the other two "S-F" dipoles are pointing "down".
Is sf4 a polar molecule
Sulfur tetrafluoride is a chemical compound with its chemical formula SF4. This compound exists as a colorless gas. It is also considered as one of the best organic fluorinating agents. Many of you may have doubts about whether SF4 is polar or nonpolar. In this article, we will check out the answer to this question and will also study its properties and its application.
Shea sweetness vip
Give their examples. Download Important Formulas pdf. Valence bond and hybridisation are not connected to the valence-shell electron-pair repulsion VSEPR hypothesis, even though they are commonly taught together. Now, whether the molecule is polar or not will depend on the orientation of all the dipoles. Oxalic-Acid vs KMnO4. In the 2p-orbitals, four hybrid orbitals overlap, whereas the fifth has just one pair. Ans : In sulphur tetrafluoride, five zones of electron density surround the core sulphur atom 4 bonds and one lone pair. Their bond dipoles do not cancel, so the molecule is polar. Access free live classes and tests on the app. JEE Application Process. So, there are four dipoles in SF 4 :. Quick links. Related articles.
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.
The central atom of sulphur tetrafluoride gains two extra electrons, giving the SF4 molecule four covalent bonds and a pair of non-bonded electrons. Therefore, the SF 4 molecule is polar. When the central atom is surrounded by bonded electron pairs and lone pairs not involved in bonding, repulsive interactions are not equivalent, and hence molecular geometry will be irregular. SF 4 molecule:. What is SF4's molecular geometry? Around sulphur, it contains one lone pair and four bonds. The easiest way to determine if a molecule is polar or nonpolar is to draw its Lewis Structure and, if necessary, check its molecular geometry. Ans : S — atom in SF Question 37d JEE Application Process. Here, the "Xe-F" bond dipoles cancel each other, so the molecule is nonpolar. Because of the 1 lone pair of electrons around the central atom, it is characterized as polar since it is more electronegative with the lone pair of electrons, and it shows how unsymmetrical it is.

I apologise, but, in my opinion, this theme is not so actual.
What magnificent phrase
And it is effective?