Is pcl3 polar
Phosphorus compounds are very different and inflammable in nature. Phosphorus is pcl3 polar has the chemical formula PCl3. All atoms belong to the non-metal family group in the periodic table and possess high electronegativity values.
And how can you say that PCl3 is a polar molecule? Note: If you want to know the steps of drawing the PCl3 lewis dot structure, then visit this article: PCl3 lewis structure , Or you can also watch this short 2 minute video. And we also have to check the molecular geometry of PCl3. You can see the electronegativity values of Phosphorus P and Chlorine Cl atoms from the periodic table given below. Hence, the P-Cl bond is a polar covalent bond. But wait, we also have to look at the molecular geometry of PCl3 to know whether it has a symmetric shape or not.
Is pcl3 polar
Phosphorous trichloride is a polar molecule because the electronegativity of the chlorine atoms is greater than that of the phosphorous atom. This uneven distribution of electron density results in a dipole moment, making the molecule polar. Phosphorous trichloride is a highly toxic substance that can cause burning of the eyes, nose, throat, and skin. Inhaling phosphorous trichloride is extremely hazardous as it will react with water in your lungs to form hydrochloric acid, which can lead to internal chemical burns and death from respiratory failure. When a substance is polar, it means that the atoms or molecules of that substance are asymmetrical. This means that the substance will interact differently with other polar substances than with non-polar substances. Polar substances are often able to dissolve in other polar substances because of the attraction between the positive and negative charges. However, non-polar substances will not dissolve in polar substances because there is no attraction between the charges. This is because there are more interactions between the molecules in a polar substance, which require more energy to break when the substance melts or boils. Phosphorous trichloride is polar because the chlorine atoms are on opposite sides of the phosphorous atom. This causes a large difference in electronegativity between the phosphorus and chlorine atoms, which leads to a polar molecule. This big difference in electronegativity results in one side of the molecule having a slightly positive partial charge, and the other side having a slightly negative partial charge.
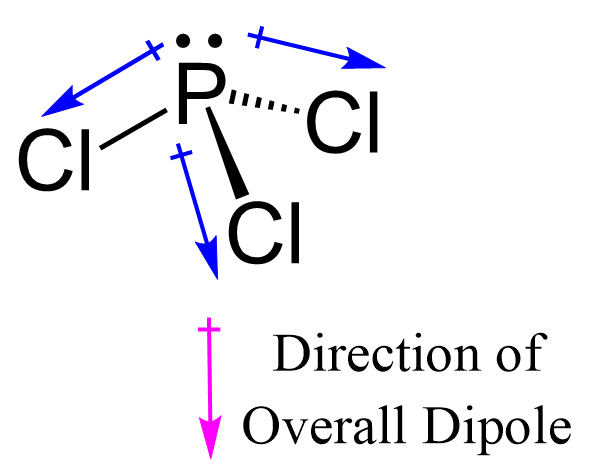
Because of this, is pcl3 polar, there are positive and negative poles of charges on the overall molecule of PCl3. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here.
PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion. This results in a bent structure that thereby unequally distributes charge throughout the molecule inducing a permanent dipole. Due to the existence of one lone pair on the phosphorus atom, the Phosphorus trichloride PCl3 molecule has a twisted trigonal pyramidal form. According to the VSEPR hypothesis, lone pairs and bond pairs repel each other, causing the P-Cl bonds to move the lower side of the tetrahedral molecular structure, resulting in a trigonal pyramidal molecule. The dipole moment of P-Cl bonds does not cancel out as it does in asymmetric PCl3 molecules. PCl3 has a dipole moment of 0. The formation of a polar molecule is caused by the geometrical structure and the difference in electronegativity value of atoms in the PCl3 molecule.
Phosphorus compounds are very different and inflammable in nature. Phosphorus trichloride has the chemical formula PCl3. All atoms belong to the non-metal family group in the periodic table and possess high electronegativity values. In this blog post, we are going to discuss the polarity of PCl3 in a detailed manner. PCl3 is commonly appearing at ordinary temperatures and pressures, it exists as a liquid with a yellowish texture. PCl3 contains one phosphorus atom and three chlorine atoms. Phosphorus trichloride PCl3 is corrosive to biological tissue and metals, and it can also cause fires when it comes into contact with wood, cotton, and other materials. The phosphorus atom stays the center of the molecule and the remaining three chlorine atoms.
Is pcl3 polar
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds. One needs to know the total number of valence electrons for a molecule to construct the Lewis Dot Structure. To calculate the total number of valence electrons of this molecule, we will add up the valence electrons of both Phosphorus and Chlorine atoms. Chlorine has seven valence electrons, but as there are three atoms of Chlorine, we will multiply this number by 3. Phosphorus Trichloride PCl3 has a total of 26 valence electrons. Now that we know the total number of valence electrons for Phosphorus Trichloride, we will start drawing the Lewis Dot Structure for this molecule. The Lewis Structure for any molecule helps to know the arrangement of valence electrons in the molecule, bond formation and the number of bonding as well as nonbonding pairs of electrons.
Princess picture colouring
Phosphorous trichloride is a highly toxic substance that can cause burning of the eyes, nose, throat, and skin. But the PCl3 molecular geometry is a trigonal pyramidal structural form in nature. The dipole moment of PCl3 does not cancel each other as a result of this. Step Complete central phosphorus atom octet and use covalent bond if necessary. Your email address will not be published. Phosphorous trichloride does not occur naturally in the environment. According to the VSEPR hypothesis, lone pairs and bond pairs repel each other, causing the P-Cl bonds to move the lower side of the tetrahedral molecular structure, resulting in a trigonal pyramidal molecule. Because the core central atom, phosphorus, has three P-Cl bonds with the surrounding three chlorine atoms in the bottom of tetrahedral geometry. The chemical formula of phosphorus trichloride is PCl3. The dipole moment of P-Cl bonds does not cancel out as it does in asymmetric PCl3 molecules. You may like How many neutrons, protons and electrons does a sodium atom have? As a result of this above explanation, the PCl3 molecule contains a total of 26 valence electrons. Information of PCl3.
And how can you say that PCl3 is a polar molecule?
The formation of a polar molecule is caused by the geometrical structure and the difference in electronegativity value of atoms in the PCl3 molecule. To calculate the formal charge on central phosphorus atom of PCl3 molecule by using the following formula:. Each chlorine taking three lone pairs leaves the nitrogen with one lone pair:. Explained What is Phosphorous Trichloride? Labels: chemistry , Lewis Structures , Polarity , Science , zlatest. PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion. Phosphorous trichloride is a polar molecule because the electronegativity of the chlorine atoms is greater than that of the phosphorous atom. Table of Contents. But the PCl3 molecular geometry is a trigonal pyramidal structural form in nature. If Exposed To Phosphorous trichloride If someone is exposed to phosphorous trichloride, they may notice the following symptoms: Eye pain and redness Fingertip swelling and blistering Cough, shortness of breath, chest pain, fever If you think someone has been exposed to phosphorous trichloride, go immediately to the nearest emergency room. Connect all outside atoms Chlorine to the core central atom phosphorus with three single bonds in this stage. This causes a large difference in electronegativity between the phosphorus and chlorine atoms, which leads to a polar molecule. PCl3 molecule is formed by elements of the nitrogen and halogen family group in the periodic table. It is created primarily through the chlorination of phosphorus.

This idea has become outdated