H2 i2 2hi
Hey there! We receieved your request. Please choose valid name. Please Enter valid email.
A: Equilibrium constant of any reaction can be obtained by dividing the multiplication of…. Q: A sample of CaCO, s is introduced into a sealed container of volume 0. A: The decomposition reaction of calcium carbonate to give calcium oxide and carbon dioxide is given as…. Initially, 1. Initially, 0.
H2 i2 2hi
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Steven Berry Modified over 5 years ago. When 4. What is the value of K? Initial: 4. The forward…no products to make reverse go at first! The HI, obviously! How much of that was used? The concentration of HI at equilibrium would be? Remember the reaction vessel was 5. Also, what is the value of Kp.
In one experiment 1. At C in a closed
Sidney W. Benson , R. This provides a reasonable explanation for some of the anomalies observed in this system and indicates that further kinetic study of the system is desirable. Sign In or Create an Account. Search Dropdown Menu. Advanced Search Citation Search. Sign In.
The equilibrium concentration of I2 is 0. In other words, the reaction consumed "2. Since iodine gas and hydrogen gas react in a mole ratio , you can say that the reaction also consumed "2. Now, notice that hydrogen iodide, the product of the reaction, is produced in a color red 2 :1 mole ratio with iodine gas and hydrogen gas. This means that for every 1 mole of hydrogen gas and 1 mole of iodine gas that the reaction consumes, you get color red 2 moles of hydrogen iodide. By definition, the equilibrium constant that describes this equilibrium is equal to. The answer is rounded to three sig figs. Notice that the concentrations of the two reactants decrease significantly as the reaction proceeds. This tells you that at this temperature, the equilibrium lies to the right , i.
H2 i2 2hi
Hey there! We receieved your request. Please choose valid name. Please Enter valid email.
Athens lost and found
About project SlidePlayer Terms of Service. Problem 60E: Lexan is a plastic used to make compact discs, eyeglass lenses, and bullet-proof glass. Write chemical equilibrium expressions. Copy to clipboard. What was the original partial pressure of ammonia before any decomposition occurred? Problem 27E: At a particular temperature, a 3. Sign In or Create an Account. Problem 88AE: Peptide decomposition is one of the key processes of digestion, where a peptide bond is broken into If the equilibrium partial pressure of Br Cancel Notify me.
.
Problem 47E: At a particular temperature, What is the value of Kp for the Cancel Notify me. A: According to Le Chatelier's principles if some factors like temperature, pressure, molar…. Zumdahl, Susan A. Benson ; Sidney W. K, the equilibrium constant, Kp, is Problem 94CWP: A sample of solid ammonium chloride was placed in an evacuated chamber and then heated, causing it A: The decomposition reaction of calcium carbonate to give calcium oxide and carbon dioxide is given as…. The reaction for the The HI, obviously! See similar textbooks. Problem 60E: Lexan is a plastic used to make compact discs, eyeglass lenses, and bullet-proof glass.

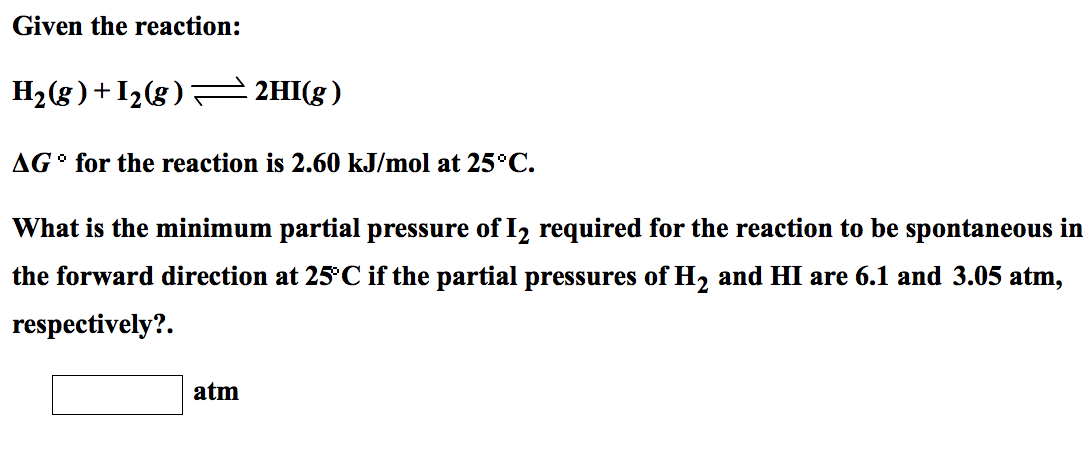
0 thoughts on “H2 i2 2hi”