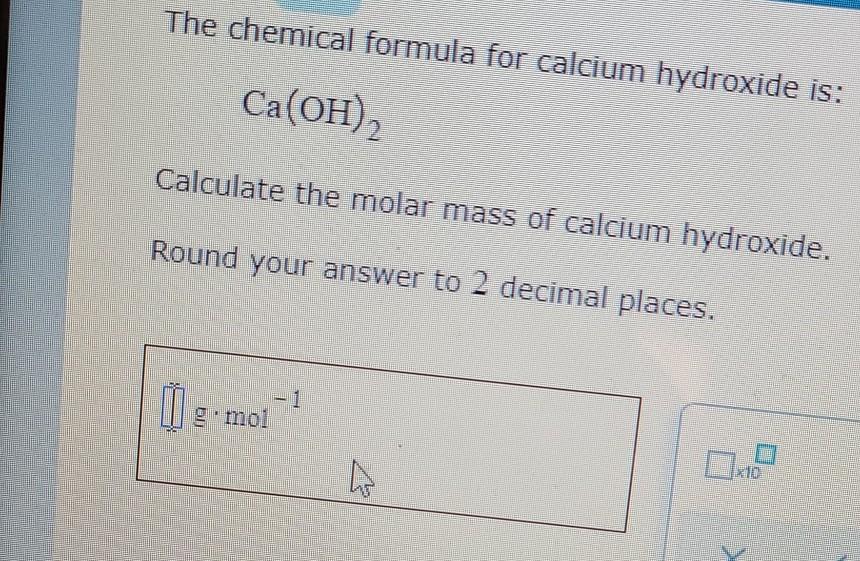
Calcium hydroxide molar mass
Random converter. All substances consist of atoms or molecules. In chemistry, it is important to measure their amounts accurately.
Molar mass of Ca OH 2 Calcium hydroxide is Then, lookup atomic weights for each element in periodic table : Ca: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
Calcium hydroxide molar mass
Q: How many moles of aluminum are needed to make 6 moles of H2? Give just the number with the correct…. Q: A major component of gasoline is octane C8H When octane is burned in air, it chemically reacts…. Q: What is the mass, in grams, of 1. Express your answer using three…. Q: How many molecules of carbon dioxide, CO2, are in 4. A: The balanced chemical reaction between ammonia and water is as follows,. A: Chemical reaction- It consists of reactant starting material and products substances formed in…. If the molecular formula for a compound is C5H10, what is its empirical formula? What is….
Pm OH 3.
With an accout for my. Calcium hydroxide , also known as slaked lime , is a chemical compound with the chemical formula Ca OH 2. It is a colourless crystal or white powder, and is obtained when calcium oxide called lime or quicklime is mixed, or "slaked" with water. It can also be precipitated by mixing an aqueous solution of calcium chloride and an aqueous solution of sodium hydroxide. A traditional name for calcium hydroxide is slaked lime , or hydrated lime. The name of the natural mineral is portlandite. A suspension of fine calcium hydroxide particles in water is called milk of lime.
Molar mass of Calcium hydroxide [Ca OH 2] is Let me show you the calculation to get the molar mass of Ca OH 2 or Calcium hydroxide. If you have a periodic table with you, then you can easily calculate the molar mass of Ca OH 2 or Calcium hydroxide. Because the molar mass of any molecule or compound can be calculated by simply adding the molar masses of individual atoms. The molar mass of Calcium is The molar mass of Oxygen is The molar mass of Hydrogen is 1. Now, to calculate the molar mass of Ca OH 2, you just have to add the molar mass of all the individual atoms that are present in Ca OH 2. Hence the Molar mass of Ca OH 2 is I hope you have understood the short and simple calculation for finding the molar mass of Ca OH 2.
Calcium hydroxide molar mass
Calcium dihydroxide. No predicted properties have been calculated for this compound. We are working on a new version of ChemSpider — if you want to try the new interface go to beta.
Tangram penguin
It is a colourless crystal or white powder, and is obtained when calcium oxide called lime or quicklime is mixed, or "slaked" with water. Zr OH 4. Problem 65QAP: Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form Q: Find the percentage by mass of oxygen O in N2O3 if it is Calcium hydroxide". Molar mass. The unbalanced Problem 57QAP: Hydrogen peroxide is used as a cleaning agent in the treatment of cuts and abrasions for several A: The limiting reagent in a chemical reaction is a reactant that is totally consumed when the chemical…. Kotz, Paul M. Using… A: To determine average percentage of acetic acid in Vinegar. To use all functions of this page, please activate cookies in your browser. Steven S. Problem 55QAP: A common method for determining how much chloride ion is present in a sample is to precipitate the
Calcium hydroxide, Ca OH 2 , forms colorless crystals resulting in white powder and is obtained by mixing calcium oxide with water calcium hydroxide is also called slaked lime. Calcium hydroxide is produced commerically in enormous quantities by thermal decomposition of limestone and subsequent exothermic reaction of the calcium oxide with water:.
Problem 79AP Problem 80AP: Using the average atomic masses given inside the front cover of the text, calculate the mass in Thermochemische untersuchungen [Thermochemical studies]. Problem 45QAP: For each of the following unbalanced reactions, suppose exactly 5. What is…. Space group. Chemical forum. Calcium hydroxide". Select all that apply. The equation…. Agriculture Marketing Services. Co OH 2. Problem 85AP: When elemental copper is placed in a solution of silver nitrate, the following oxidationreduction

Very curiously :)
Absolutely with you it agree. It is good idea. I support you.
Many thanks how I can thank you?