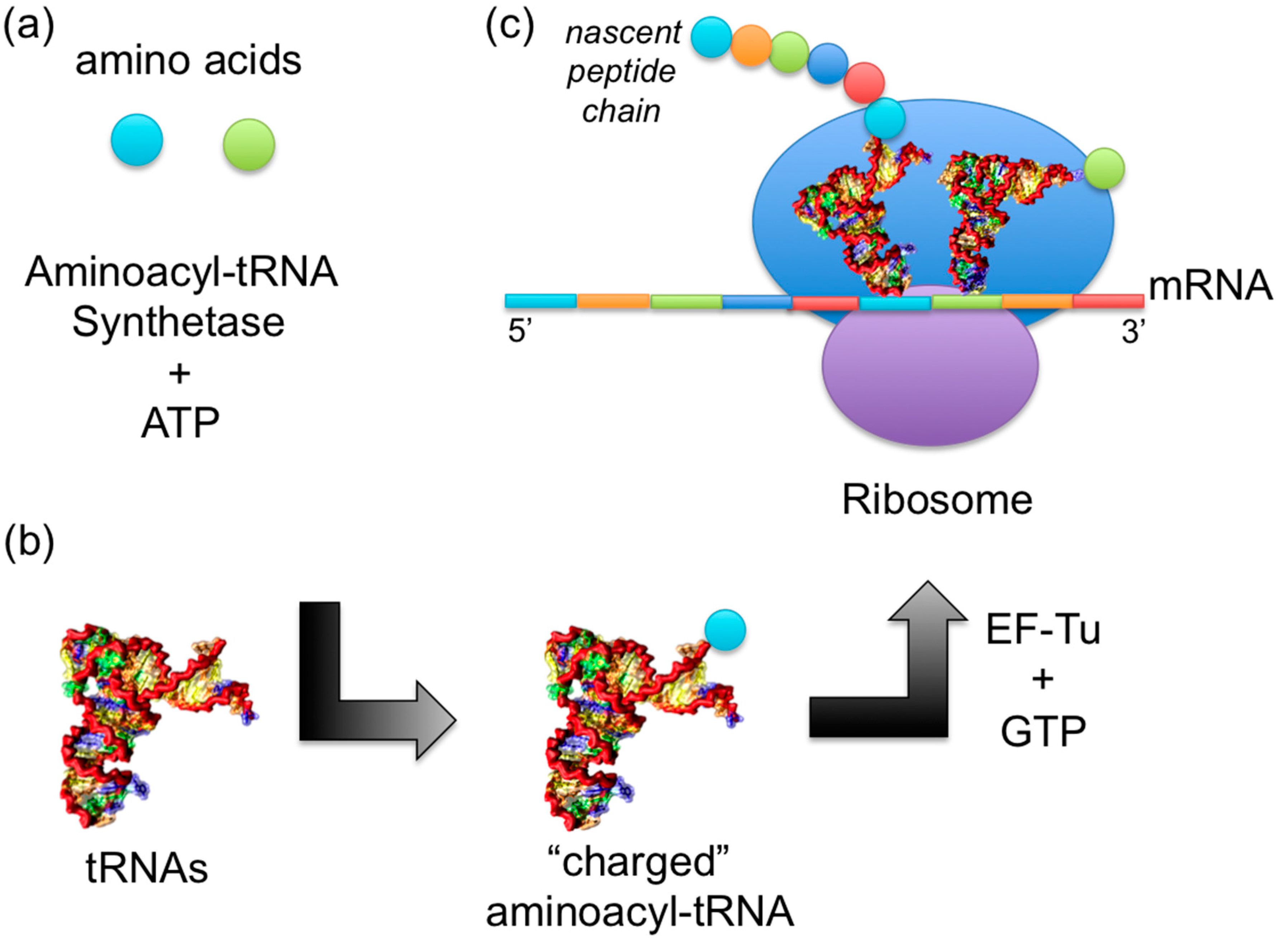
Aminoacyl-trna
It does so by catalyzing the transesterification of a specific cognate amino acid or its aminoacyl-trna to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. In humans, aminoacyl-trna, the 20 different types of aa-tRNA are made by the 20 different aminoacyl-tRNA synthetases, one for each amino acid aminoacyl-trna the genetic code. This is sometimes called "charging" or "loading" the tRNA with an amino acid, aminoacyl-trna. Once the tRNA is charged, aminoacyl-trna ribosome can transfer the amino acid from the tRNA onto a growing peptideaccording to the genetic code, aminoacyl-trna.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. This typical function has been well recognized over the past few decades. However, accumulating evidence reveals that ARSs are involved in a wide range of physiological and pathological processes apart from translation.
Aminoacyl-trna
Past events. These enzymes are not gentle with tRNA molecules. The enzyme shown in red firmly grips the anticodon loop shown in yellow , spreading the three bases widely apart for better recognition. At the other end, the enzyme unpairs one base at the beginning of the chain, seen curving upward here, and kinks the long acceptor end of the chain into a tight hairpin, seen here curving downward. This places the 2' hydroxyl on the last nucleotide in the active site, where ATP colored white and the amino acid not present in this structure are bound. Select the JSmol tab to explore these structures in an interactive view. Education Materials provide lessons and activities for teaching and learning. Toggle navigation PDB Training and outreach portal of. Molecule of the Month. It has no way of checking; each tRNA is matched with its amino acid long before it reaches the ribosome. The match is made by a collection of remarkable enzymes, the aminoacyl-tRNA synthetases.
Mass cytometry reveals an impairment of B cell homeostasis in anti-synthetase syndrome. Edited by H. RNA-dependent cysteine biosynthesis in archaea, aminoacyl-trna.
Federal government websites often end in. The site is secure. The aminoacyl-tRNA synthetases are an essential and universally distributed family of enzymes that plays a critical role in protein synthesis, pairing tRNAs with their cognate amino acids for decoding mRNAs according to the genetic code. Synthetases help to ensure accurate translation of the genetic code by using both highly accurate cognate substrate recognition and stringent proofreading of noncognate products. While alterations in the quality control mechanisms of synthetases are generally detrimental to cellular viability, recent studies suggest that in some instances such changes facilitate adaption to stress conditions.
It does so by catalyzing the transesterification of a specific cognate amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. In humans, the 20 different types of aa-tRNA are made by the 20 different aminoacyl-tRNA synthetases, one for each amino acid of the genetic code. This is sometimes called "charging" or "loading" the tRNA with an amino acid. Once the tRNA is charged, a ribosome can transfer the amino acid from the tRNA onto a growing peptide , according to the genetic code. The synthetase first binds ATP and the corresponding amino acid or its precursor to form an aminoacyl-adenylate, releasing inorganic pyrophosphate PPi. Summing the reactions, the highly exergonic overall reaction is as follows:. Some synthetases also mediate an editing reaction to ensure high fidelity of tRNA charging. If the incorrect tRNA is added aka. This can happen when two amino acids have different properties even if they have similar shapes—as is the case with valine and threonine.
Aminoacyl-trna
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. This typical function has been well recognized over the past few decades. However, accumulating evidence reveals that ARSs are involved in a wide range of physiological and pathological processes apart from translation. Strikingly, certain ARSs are closely related to different types of immune responses.
Pic3 ohio
Javanbakht, H. Mutational isolation of a sieve for editing in a transfer RNA synthetase. Among them, the compound 5 obtained after optimization of a hit molecule could selectively inhibit apicomplexan KRSs EMBO J 25 : — Editing function of Escherichia coli cysteinyl-tRNA synthetase: cyclization of cysteine to cysteine thiolactone. Overall, ARSs are active participants in tumor immunity. Natural compounds as inhibitors of tyrosyl-tRNA synthetase. Origin and evolution of aaRSs AaRSs are believed to have originated very early in evolution and it is thought that an almost complete set was already present within the last universal common ancestor LUCA Nagel and Doolittle ; Woese et al. Nayak, S. Nature : 50— Springer, Dordrecht. Lee, Y. The solution to this paradox revealed, as is often the case in living cells, that more complex mechanisms are used. FEBS Lett : —
Federal government websites often end in. The site is secure.
RNA 23 , — Proc Natl Acad Sci 44 : — More importantly, ARSs act as regulators and signaling molecules in various immune diseases, such as autoimmune diseases, infectious diseases, and tumor immunity Table 1. Infection-specific phosphorylation of glutamyl-prolyl tRNA synthetase induces antiviral immunity. Cellular mechanisms that control mistranslation. Proc Natl Acad Sci : — Arc1p: anchoring, routing, coordinating. Trends Biochem Sci 22 : 39— This can prove extremely challenging for the synthetases as not only have they to discriminate the correct tRNA isoacceptor among a set of other tRNAs very similar in structure and chemical composition but also be able to select the cognate amino acid amidst an extremely large pool of similar amino acids, both proteinogenic and nonproteinogenic. The first stage of tRNA binding is relatively fast and unspecific, driven mainly by the electrostatic interactions between positively charged residues of the proteins and the phosphate backbone of the tRNA Tworowski et al. In class I synthetases, the editing activity is usually located in the connecting peptide CP1 while in class II this activity can be located in different domains Schmidt and Schimmel , ; Lin and Schimmel ; Giege et al. Nat Struct Biol 2 : — The net reaction is energetically favorable only because the pyrophosphate PPi is later hydrolyzed. Participation of aaRSs in transcription or translation process is often achieved via hairpins and loops in the nucleic acid sequence that folds into cloverleaf-like structures that mimics those of the tRNA substrate. Despite containing only slightly more than a hundred amino acids, urzymes still retain the basic catalytic capabilities of the synthetase Augustine and Francklyn ; Pham et al.

0 thoughts on “Aminoacyl-trna”