Why was periactin taken off the market
Cyproheptadinesold under the brand name Periactin among others, is a first-generation antihistamine with additional anticholinergicantiserotonergicfabswimgers local anesthetic properties. It was patented in and came into medical use in
A drug recall is the most effective way to protect the public from a defective or potentially harmful product. A recall is a voluntary action taken by a company to remove a defective drug product from the market or warn patients and consumers about a potential risk. A Terminated Recall is a recall where the FDA has determined that all reasonable efforts have been made to remove or correct the violative product in accordance with the recall strategy, and proper disposition has been made according to the degree of hazard. Recalls that are not indicated as being terminated are either ongoing or completed. The list below includes voluntary drug recalls in which public notification has been issued. Filter by. Terminated Recall - Any - Yes No.
Why was periactin taken off the market
Federal government websites often end in. The site is secure. Objectives: Cyproheptadine is a first-generation H1-antihistamine drug first that was distributed in the s. While its orexigenic effect was observed early, cyproheptadine is not yet authorized for this indication in all countries today. There is an increasing medical interest and demand for the orexigenic effect of cyproheptadine, especially in children with poor appetite. As cyproheptadine might be evaluated in future clinical trials, we wanted to assess its safety profile. Methods: Using the French national pharmacovigilance database, we retrospectively analyzed all pediatric and adult reports of adverse effects of cyproheptadine recorded since its first distribution in France. Next, we performed a systematic review of the literature of cyproheptadine adverse effects. Results: Since , 93 adverse effects were reported in the French pharmacovigilance database adults In the literature, the most frequent adverse effect reported was drowsiness in adults or children, and five case reports noted liver complications in adults. We estimated the frequency of hepatic adverse effects at 0. Conclusion: Cyproheptadine can be considered a safe drug. Mild neurological effects appear to be frequent, and hepatotoxicity is uncommon to rare. Randomized controlled trials are needed to evaluate the safety and efficacy of cyproheptadine before authorization for appetite stimulation, especially in young children as studies at this age are lacking. Possible hepatic complications should be monitored, as very rare cases of liver failure have been reported.
Int J Mol Sci. Take cyproheptadine at around the same time s every day.
Official websites use. Share sensitive information only on official, secure websites. Cyproheptadine relieves red, irritated, itchy, watery eyes; sneezing; and runny nose caused by allergies, irritants in the air, and hay fever. It may also be used to relieve the itching of allergic skin conditions, and to treat hives, including hives caused by exposure to cold temperatures and by rubbing the skin. Cyproheptadine is also sometimes used to treat allergic reactions in people who have received blood products as part of medical treatment and to treat life-threatening allergic reactions after the symptoms have been brought under control with other medications. Cyproheptadine will help relieve symptoms but will not treat the cause of symptoms or speed recovery. Cyproheptadine is in a class of medications called antihistamines.
Cyproheptadine is an antihistamine that reduces the effects of natural chemical histamine in the body. Histamine can produce symptoms of sneezing, itching, watery eyes, and runny nose. Cyproheptadine is used to treat sneezing, runny nose, itching, red or watery eyes, and other symptoms of seasonal allergies hay fever. Cyproheptadine is also used to treat other conditions such as eczema or skin reactions to insect bites. Cyproheptadine is sometimes used to treat certain types of headaches, including migraines. Cyproheptadine may also be used for purposes not listed in this medication guide. Do not give this medicine to a newborn or premature baby.
Why was periactin taken off the market
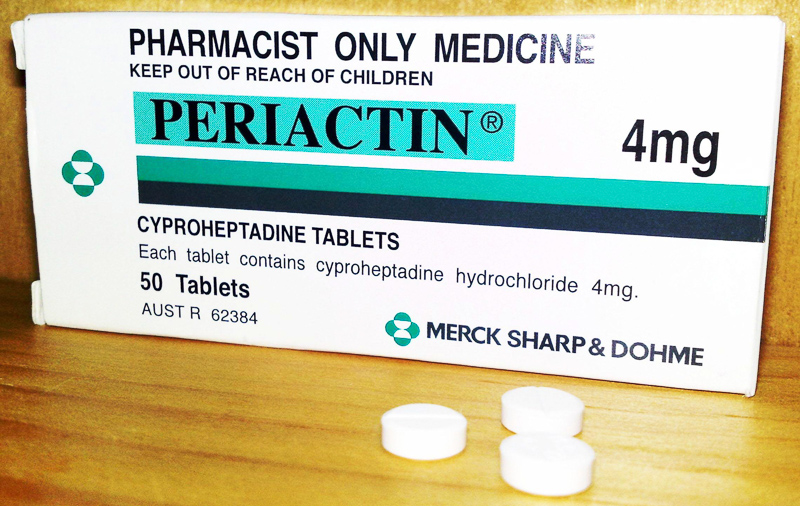
Cyproheptadine , sold under the brand name Periactin among others, is a first-generation antihistamine with additional anticholinergic , antiserotonergic , and local anesthetic properties. It was patented in and came into medical use in Cyproheptadine is used to treat allergic reactions specifically hay fever. It is also used as a preventive treatment against migraine. In a study the frequency of migraine was dramatically reduced in patients within 7 to 10 days after starting treatment. The average frequency of migraine attacks in these patients before administration was 8. It is also used off-label in the treatment of cyclical vomiting syndrome in infants; the only evidence for this use comes from retrospective studies. Cyproheptadine is sometimes used off-label to improve akathisia in people on antipsychotic medications. It is used off-label to treat various dermatological conditions, including psychogenic itch [13] drug-induced hyperhidrosis excessive sweating , [14] and prevention of blister formation for some people with epidermolysis bullosa simplex. One of the effects of the drug is increased appetite and weight gain, which has led to its use off-label in the USA for this purpose in children who are wasting as well as people with cystic fibrosis.
60 second ps4
Blockers Amiloride Benzamil Triamterene. VA conceptualized and designed the study, analyzed data, performed the literature search, reviewed and revised the manuscript. Causality assessment in pharmacovigilance: the French method and its successive updates. Undeclared Tianeptine. Side effects of cyproheptadine. Appetite stimulants A Cyproheptadine has been studied for the treatment of post-traumatic stress disorder. Daridorexant Lemborexant Suvorexant. Call your doctor if you have any unusual problems while you are taking this medication. Central anticholinergic syndrome on therapeutic doses of cyproheptadine. J Pharmacol Sci. S2CID All AEs appeared within a few days after that start of cyproheptadine treatment.
.
Aspen Pharmacare Australia. We found that mild neurological effects were frequent, and that hepatotoxicity was uncommon to rare. This medication may be prescribed for other uses. Miscellaneous: Prinzmetal angina, parasitological diseases, blepharospasm, cerebral vasoconstriction syndrome. Antagonists: Metitepine methiothepin. Categories : Antiandrogens Antihistamines Antipsychotics Appetite stimulants Dibenzocycloheptenes Dopamine antagonists Local anesthetics Muscarinic antagonists Piperidines 5-HT2 antagonists Serotonin receptor antagonists Sodium channel blockers. Cyproheptadine may cause side effects. Do not take a double dose to make up for a missed one. Double-blind comparative study of treatment with cyproheptadine, chlorpheniramine, and placebo". A retrospective review of cyproheptadine for feeding intolerance in children less than three years of age: effects and side effects. Int J Immunopathol Pharmacol. It is important to keep all medication out of sight and reach of children as many containers such as weekly pill minders and those for eye drops, creams, patches, and inhalers are not child-resistant and young children can open them easily. ISBN

I join. I agree with told all above. Let's discuss this question. Here or in PM.