Why is graphite a good conductor of electricity
Graphite is a good conductor of both electricity and heat. This is due to its molecular structure, which allows electrons to move freely through it. Rather than loosen one sheet of molecules from another, you have to break the covalent bonding the particular way in which atoms are bonded together throughout the whole structure in order to melt the material. Substances like graphite which have these giant structures have very high melting points.
Use app Login. Why is graphite a good conductor of electricity and a good lubricant? Explain with the help of diagram. Open in App. Verified by Toppr. Each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array.
Why is graphite a good conductor of electricity
Graphite is a good conductor of heat and electricity because it contains. Therefore, graphite is a good conductor of heat and electricity because it contains free electrons which can conduct heat and electricity. Byju's Answer. Open in App. Conduction of heat and electricity: Graphite is the crystalline allotropes of carbon and it has a layered structure. Conduction of heat and electricity in a substance occurs due to the presence of free electrons in that material. Only three or four valence electrons of each carbon atom are involved in bonding, so, each carbon atom makes use of sp 2 hybrid orbitals, Thus the fourth valence electron of each carbon atom remains unpaired. This free electron is responsible for the conduction of heat and electricity. Graphite is a good conductor of heat and electricity because it contains :. Graphite is a good conductor of electricity because it has:. Graphite is a good conductor of electricity because:. Graphite is a good conductor of electricity due to?
Answer: the very reason why metals do.
Answer: the very reason why metals do. As she points out, graphite is made from carbon atoms, which have four electrons in their outer shells. Importantly, the layers in graphite are 'aromatic'. Rather than suggesting they smell nice, this means the atoms in a single layer have alternating single and double bonds see diagram below. This doesn't only strengthen graphite's structure but allows electrons to move freely along the layers. In other materials, there may not be free cars to make this journey, meaning they're not conductive. True, both diamonds and graphite are made from carbon.
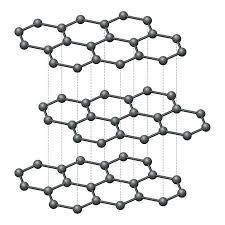
Graphite is a fascinating material that has garnered significant attention for its unique properties, particularly its ability to conduct electricity. In this article, we will delve into the reasons why graphite is such a good conductor of electricity, exploring its molecular structure, applications in various industries, and the implications for future technological advancements. Graphite is composed of carbon atoms arranged in a hexagonal lattice, forming layers of interconnected sheets. These sheets are held together by weak van der Waals forces, allowing them to easily slide past each other. This structure gives graphite its characteristic slippery feel and also enables the flow of electrons through the material. In graphite, each carbon atom is bonded to three other carbon atoms, leaving one electron free to move within the material. These delocalized electrons are not confined to a specific bond, allowing them to flow freely through the layers of graphite, carrying electrical current along the way. Due to its excellent conductivity, graphite finds a wide range of applications in various industries. Some of the most common uses of graphite as a conductor include:.
Why is graphite a good conductor of electricity
It is a naturally occurring mineral that is found in metamorphic and igneous rocks. Diamond and Graphite both are mineral of carbon having the same composition but with different chemical structures. Graphite has properties of both metal and non-metal which make it an interesting element. Many students may have a question about whether graphite conducts electricity or not. So, In this article, I will answer this question and cover the surrounding topics too. So, does graphite conduct electricity? Yes, graphite is a very good conductor of electricity because of delocalized electrons. Graphite forms layers of a hexagonal arrangement of atoms and each carbon atom form a covalent bond with three other carbon atoms, and therefore each carbon atom has one non-bonded electron which becomes delocalized and responsible for conducting electricity.
Is remble still alive
Graphite is a refractory mineral, which means it is stable over a wide range of temperatures and able to retain its strength and form at very high temperatures. Each carbon atom is then bound weakly to two other carbon atoms, one to the sheet above it and another to the sheet below it. Because these layers are weakly bonded together, they slide over each other easily. However, diamonds are one of the hardest materials known to man, while graphite is not. Verified by Toppr. Diamond is hard, but very brittle. Due to this these layers can slip over each other so graphite has a slippery surface. Resistance to thermal shock Thermal shock is when a sudden change in temperature—hot to cold, cold to hot—puts tension on a material, causing it to break. This is what makes graphite a soft and slippery material and gives it its self-lubricating properties. This is due to its molecular structure, which allows electrons to move freely through it.
Answer: the very reason why metals do.
Rather than suggesting they smell nice, this means the atoms in a single layer have alternating single and double bonds see diagram below. Therefore, out of the four valence electrons in a carbon atom, only three are used for bonding and the fourth is relatively free and can move from one carbon atom to the other. Graphite is a good conductor of both electricity and heat. Open in App. Her current research focuses on building materials to be used in the safe operation of nuclear reactors. Both diamonds and graphite have extremely high melting points — both above degrees Celcius. View More. Graphite reduces friction and noise from machinery and keeps equipment running without issue for longer periods of time. Conduction of heat and electricity: Graphite is the crystalline allotropes of carbon and it has a layered structure. However, their structures are significantly different. This doesn't only strengthen graphite's structure but allows electrons to move freely along the layers. Graphite Mould. Thermal shock is when a sudden change in temperature—hot to cold, cold to hot—puts tension on a material, causing it to break. Graphite is a good conductor of heat and electricity because it contains. Graphite is closely related to diamonds.

You are mistaken. I can prove it. Write to me in PM, we will talk.
It cannot be!