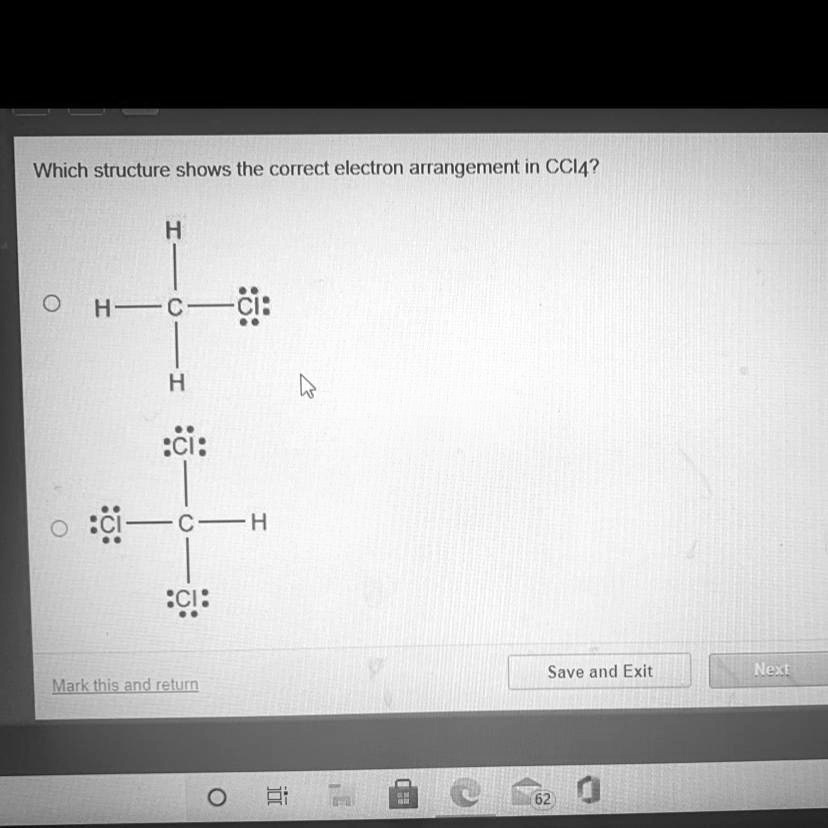
Which structure shows the correct electron arrangement in ccl4
Up until this point, we've been determining the types of hybrid orbitals by examining pictures of each molecule being studied.
Submitted by Victor M. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Draw the Lewis structure and compute for the formal charge for each atom in CCl4. Which structure shows the correct electron arrangement in CCl4?
Which structure shows the correct electron arrangement in ccl4
Draw a skeleton structure in which the other atoms are single-bonded to the central atom — a "C" atom with four "Cl" atoms attached to it. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Every atom in the trial structure has an octet. Count the valence electrons in your trial structure Count the valence electrons you actually have available. The trial structure has exactly the same number of electrons as we have available. The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible. What is the molecular geometry of CCl4? Ernest Z. Jun 14, Decide which atom is the central atom in the structure. That will be the least electronegative atom "C". Thus, the trial structure is the correct Lewis structure. The bonds will emanate from the central atom at angles of
Each chlorine atom needs 6 more electrons, so we will use all 24 electrons.
Submitted by Angela G. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. What type of electron group arrangement is created from an sp3d hybridized orbital set?
Draw a skeleton structure in which the other atoms are single-bonded to the central atom — a "C" atom with four "Cl" atoms attached to it. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Every atom in the trial structure has an octet. Count the valence electrons in your trial structure Count the valence electrons you actually have available. The trial structure has exactly the same number of electrons as we have available. The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible. What is the molecular geometry of CCl4? Ernest Z.
Which structure shows the correct electron arrangement in ccl4
Carbon and chlorine, being non-metals, form a molecular compound by sharing electrons. Begin by determining the total number of valence electrons. Carbon group 14 brings four valence electrons, and there are four chlorine atoms group 17 , each contributing seven valence electrons. Recognize that carbon will be the central atom.
Imagenes de gatitos aesthetic
Polarity overview University of Ari… General Chemistry…. Count the valence electrons in your trial structure Unfortunately, randomness rarely yields the right answer, so we'll need some rules to help us out. Subtract the number of valence electrons from the number of octet electrons to find the number of electrons that are involved in bonding. Already have an account? Count the valence electrons you actually have available. What type of electron group arrangement is created from an sp3d hybridized orbital set? Question 2a64e. A central upper C is single bonded to upper C l to the top, bottom, and left; those upper C l atoms have three pairs of electron dots on the sides away from the bond. In this step, it's tempting to just randomly stick atoms and bonds wherever you can until everything is stuck together. Since chlorine wants eight electrons, three pairs need to be added to each. Chlorine Cl has 7 valence electrons. Try Numerade free for 7 days View This Answer.
The Lewis structure of CCl4, also known as carbon tetrachloride, is a representation of how the atoms are arranged in the molecule.
See also:. A central upper C is single bonded to upper H above, below, and to the left, and single-bonded to upper C l to the right. Chem - coursework University of Ill… Organic Chemistry…. Ask unlimited questions and get video answers from our expert STEM educators. Ionic Nomenclature. So we developed a line of study tools to help students learn their way. Answer 5. The C l has three pairs of electron dots, above, right, and below. The number of "octet electrons" is equal to the number of valence electrons that each atom will have when they have the same electron configuration as the nearest noble gas the octet rule. Thanks for the breakdown of the correct electron arrangement in CCl4 Really helped to clarify things. View More Comments. Draw a skeleton structure in which the other atoms are single-bonded to the central atom — a "C" atom with four "Cl" atoms attached to it.

I am final, I am sorry, would like to offer other decision.
I have thought and have removed the idea
I think, that you are not right. Write to me in PM, we will discuss.