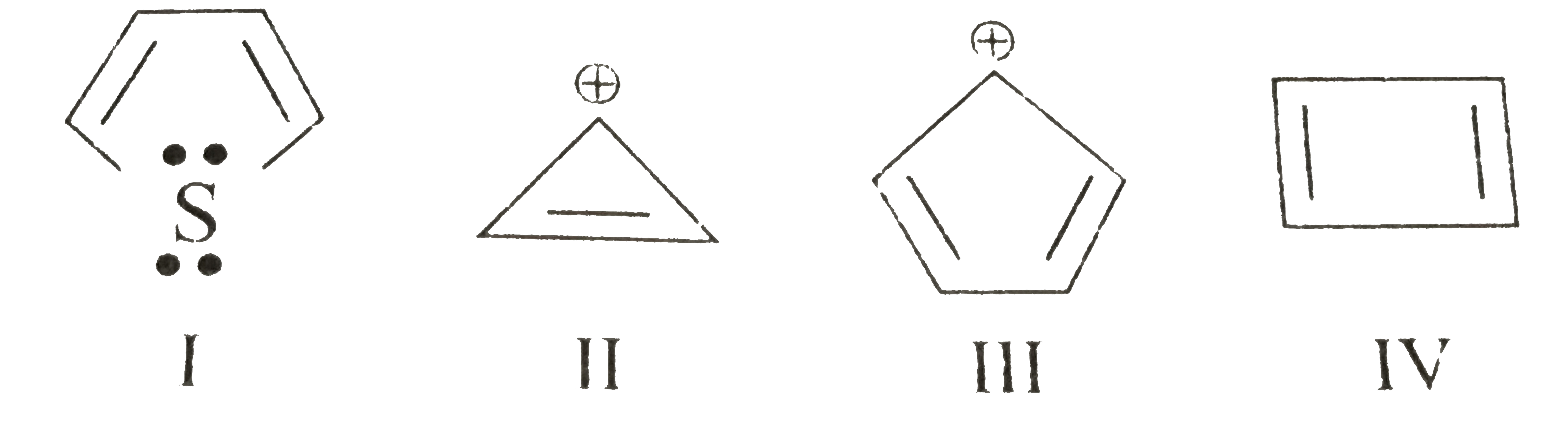
Which of the following is antiaromatic
In contrast to the diamagnetic ring current present in aromatic compoundsantiaromatic compounds have a paramagnetic ring current, which can be observed by NMR spectroscopy. Examples of antiaromatic compounds are pentalene Abiphenylene Bcyclopentadienyl cation C.
Hence, is anti-aromatic. Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam.
Which of the following is antiaromatic
Which of the following is an antiaromatic compound? Which of the following species are antiaromatic? Which of the following are pairs of antiaromatic species? Which of the following species is antiaromatic? Consider the following reactions : Which of the following are stereospecific reactions? Antidepressant drugs follow which of the following mechanism? Sulphur, on heating follows Which of the following sequence? Match the following: Which of the following is the best matched options? Which of the followig is aromatic? Which of the following is not aromatic? Which of the followng is the least reactive towards addition reactions The order of stability of the species i , ii and iii is. In which of the following compounds are the hydrogen atoms atoms of th Which of the following is expected to be the least stable? Which of the followig is not aromatic?
BOB Acquisition Officer. Speed of sound is highest in which medium?
.
Some Practice Problems. Antiaromatic Compounds and Antiaromaticity. Our last post in this series on aromaticity went through the 4 conditions a molecule must fulfill in order to be aromatic. In that post we tried to explain what each of those rules meant — so if any of these individual items seem unclear to you, it might be a good idea to go back to that post. Make a table. You need to know the few exceptions that come up — we covered that last time. The easiest example to start with is benzene, and it demonstrates how to use the table.
Which of the following is antiaromatic
We have seen that aromatic compounds are cyclic, planar and have a fully conjugated system of orbitals which gives them a special stability. There is, however, one more criterion that compounds must match in order to be aromatic. Not all the compounds that are cyclic, planar, and fully conjugated are aromatic.
Pdf all the young dudes
RBI Office Attendant. Navy MR Agniveer. Punjab Superior Judicial Service. TN TET. Telangana High Court Copyist. Cochin Shipyard Executive Trainee. Rajasthan High Court Civil Judge. CTU Conductor. Rajasthan PTET. RBI Grade B. C iii and iv. Cyclooctatetraene is Telangana High Court Junior Assistant. BDL Management Trainee. Anti- aromatic compounds are unstable.
If a compound does not have a continuous ring of conjugated p orbitals in a planar conformation, then it is nonaromatic. Huckel's Rule is a useful first step in evaluating the potential for a ringed molecule to be aromatic. The planar requirement of the ring may require further investigation.
CG Vyapam SI. Central Bank Apprentice. Allahabad High Court Group D. Maharashtra Zilla Parishad Extension Officer. BIS Senior Technician. Punjab Police Jail Warder. Vizag Steel Trade Apprentice. Goa Police Sub Inspector. NVS Staff Nurse. Rajasthan High Court Civil Judge.

This theme is simply matchless :), it is interesting to me)))
Remarkable question