What is the specific heat of a substance
When summer hits, you might end up going to the beach to cool down. While the ocean waves may feel cool, the sand, unfortunately, is red-hot.
Heat capacity is an extensive property, so it scales with the size of the system. For example, if it takes 1, J to heat a block of iron, it would take 2, J to heat a second block of iron with twice the mass as the first. The heat capacity of most systems is not a constant. Rather, it depends on the state variables of the thermodynamic system under study. In particular, it is dependent on temperature itself, as well as on the pressure and the volume of the system, and the ways in which pressures and volumes have been allowed to change while the system has passed from one temperature to another.
What is the specific heat of a substance
If a swimming pool and wading pool, both full of water at the same temperature, were subjected to the same input of heat energy, the wading pool would certainly rise in temperature more quickly than the swimming pool. The heat capacity of an object depends both on its mass and its chemical composition. Because of its much larger mass, the swimming pool of water has a larger heat capacity than the wading pool. Different substances respond to heat in different ways. If a metal chair sits in the bright sun on a hot day, it may become quite hot to the touch. An equal mass of water under the same sun exposure will not become nearly as hot. Water is very resistant to changes in temperature, while metals generally are not. The table below lists the specific heats of some common substances. Notice that water has a very high specific heat compared to most other substances. Water is commonly used as a coolant for machinery because it is able to absorb large quantities of heat see table above. Coastal climates are much more moderate than inland climates because of the presence of the ocean. Water in lakes or oceans absorbs heat from the air on hot days and releases it back into the air on cool days. Search site Search Search. Go back to previous article. Sign in.
Specific heat capacity often varies with temperature, and is different for each state of matter. In theory, the specific heat capacity of a substance can also be derived from its abstract thermodynamic modeling by an equation of state and an internal energy function. Oxford University Press.
In thermodynamics , the specific heat capacity symbol c of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity or as the specific heat. Specific heat capacity often varies with temperature, and is different for each state of matter. The specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is heated specific heat capacity at constant pressure than when it is heated in a closed vessel that prevents expansion specific heat capacity at constant volume. Specific heat capacity is also related to other intensive measures of heat capacity with other denominators. One of the first scientists to use the concept was Joseph Black , an 18th-century medical doctor and professor of medicine at Glasgow University. He measured the specific heat capacities of many substances, using the term capacity for heat.
If a swimming pool and wading pool, both full of water at the same temperature, were subjected to the same input of heat energy, the wading pool would certainly rise in temperature more quickly than the swimming pool. The heat capacity of an object depends both on its mass and its chemical composition. Because of its much larger mass, the swimming pool of water has a larger heat capacity than the wading pool. Different substances respond to heat in different ways. If a metal chair sits in the bright sun on a hot day, it may become quite hot to the touch.
What is the specific heat of a substance
In equation form, this can be represented as the following:. That is if a constant has units, the variables must fit together in an equation that results in the same units. So C equals something with energy in the numerator and temperature in the denominator. Now, you need to use some common sense here, as we are adding heat, not work, and adding heat changes the temperature, it does not make the temperature. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly.
100 روبل روسي بكام مصري
The polytropic heat capacity is calculated at processes if all the thermodynamic properties pressure, volume, temperature change. Calorimetry is used to measure the amount of heat produced or consumed in a chemical reaction. Note that the total heat capacity C is simply the product of the specific heat capacity c and the mass of the substance m, i. The heat capacity of an object depends both on its mass and its chemical composition. Shields translation 2 ed. Heat Capacity and Specific Heat Different substances respond to heat in different ways. The specific heat capacity can be defined and measured for gases, liquids, and solids of fairly general composition and molecular structure. We'll assume you're ok with this, but you can opt-out if you wish. Explore our app and discover over 50 million learning materials for free. For gases, and also for other materials under high pressures, there is need to distinguish between different boundary conditions for the processes under consideration since values differ significantly between different conditions. The specific heat capacity of a substance is typically determined according to the definition; namely, by measuring the heat capacity of a sample of the substance, usually with a calorimeter , and dividing by the sample's mass. The Measurement of Heat Capacity The heat capacity of most systems is not a constant.
The learning objectives in this section will help your students master the following standards:. If two objects at different temperatures are brought in contact with each other, energy is transferred from the hotter object that is, the object with the greater temperature to the colder lower temperature object, until both objects are at the same temperature.
Measuring the specific heat capacity at constant volume can be prohibitively difficult for liquids and solids. Wikimedia Commons. The specific heat capacity can be defined and measured for gases, liquids, and solids of fairly general composition and molecular structure. Low temperature approximations for both gases and solids at temperatures less than their characteristic Einstein temperatures or Debye temperatures can be made by the methods of Einstein and Debye discussed below. The path integral Monte Carlo method is a numerical approach for determining the values of heat capacity, based on quantum dynamical principles. The word calorimetry is derived from the Latin word calor , meaning heat. False True. It does not account for the heat loss through the container or the heat capacity of the thermometer and container itself. Necessary cookies are absolutely essential for the website to function properly. As you can see, water has a different specific heat when it is a solid, liquid, and gas. In order to do calorimetry, it is crucial to know the specific heats of the substances being measured. On the other hand, measuring the specific heat capacity at constant volume can be prohibitively difficult for liquids and solids, since one often would need impractical pressures in order to prevent the expansion that would be caused by even small increases in temperature. Provided by : Boundless Learning. Since they cannot be calculated easily, they are empirically measured and available for reference in tables. Coastal climates are much more moderate than inland climates because of the presence of the ocean.

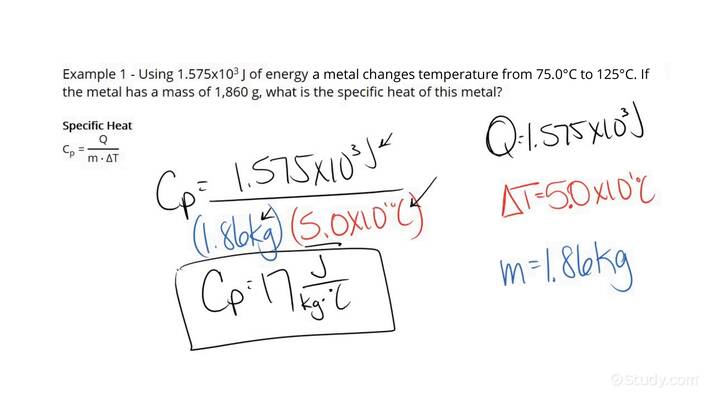
I think, that you are mistaken. I can prove it. Write to me in PM, we will communicate.
What entertaining answer
What remarkable words