What is the mass of precipitate formed
Clearly the reagents are present in molar ratio. The reaction that occurs in solution is the precipitation of a curdy white mass of AgCl si. And given the stoichiometrywe gets 0. Of course a material such as silver halide would be very hard to isolate.
We endeavor to keep you informed and help you choose the right Career path. When you look back in life , this app would have played a huge role in laying the foundation of your career decisions. Found everything I wanted and it solved all of my queries for which I was searching a lot A must visit No need to find colleges in other sites, this is the best site in India to know about any colleges in India. Home QnA Home what is the mass of precipitate formed when 50mL of Get answers from students and experts Ask.
What is the mass of precipitate formed
Q: A What is the mass, in…. Q: Many metal ions are precipitated from a solution by the sulfide ion. As an example, consider…. Q: Barium hydroxide is mixed with lithium phosphate at room temperature. If Q: Many metal ions are precipitated from solution by the sulfide ion. As an example, consider treating…. Q: A student weighs out a 1. Q: 10 mL of a 30 M sodium phosphate solution…. A: The mass of lead phosphate precipitate is calculated as,. Q: A student adds KF to mL of a 0.
Then a solution of the chloride was treated with Molecular Geometry.
Determine the i identity and ii mass of the precipitate formed when a Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties.
Chemistry Glossary Definition of Precipitate. In chemistry, to precipitate is to form an insoluble compound either by reacting two salts or by changing the temperature to affect the solubility of the compound. Precipitation may indicate a chemical reaction has occurred, but it may also occur if a solute concentration exceeds its solubility. Precipitation is preceded by an event called nucleation, which is when small insoluble particles aggregate with each other or else form an interface with a surface, such as the wall of a container or a seed crystal. The terminology can seem a bit confusing. Here's how it works: forming a solid from a solution is called precipitation. A chemical that causes a solid to form in a liquid solution is called a precipitant.
What is the mass of precipitate formed
Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. Because not all aqueous reactions form precipitates, one must consult the solubility rules before determining the state of the products and writing a net ionic equation. The ability to predict these reactions allows scientists to determine which ions are present in a solution, and allows industries to form chemicals by extracting components from these reactions.
Pornhub con
Label the acid, base, and salt. Q: Calculate the Molarity when Periodic Trend: Ionization Energy. Q: Calculate the mass, in grams, of potassium iodide that must be added to a mL volumetric flask in… A:. For oversatureated solutions, Q sp is greater than K sp. Henry's Law Calculations. Calculate the molarity of the solution. Are you sure you want to delete your answer? Precipitation of ceramic phases in metallic alloys such as zirconium hydrides in zircaloy cladding of nuclear fuel pins can also render metallic alloys brittle and lead to their mechanical failure. Because the reactants are ionic and aqueous, they dissociate and are therefore soluble. Addition and Subtraction Operations. Face Centered Cubic Unit Cell.
A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. We described a precipitation reaction in which a colorless solution of silver nitrate was mixed with a yellow-orange solution of potassium dichromate to give a reddish precipitate of silver dichromate:. Thus precipitation reactions are a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble.
Treatment of the sample with Na2CO3 produces a precipitate, but treatment with ammonium sulfate does not. Thermal Equilibrium. Question cannot be greater than characters. Related Questions. Steven S. It is a…. Problem 69QAP: A student is asked to identify the metal nitrate present in an aqueous solution. Redox Reactions. Periodic Trend: Effective Nuclear Charge. Gas Evolution Equations.

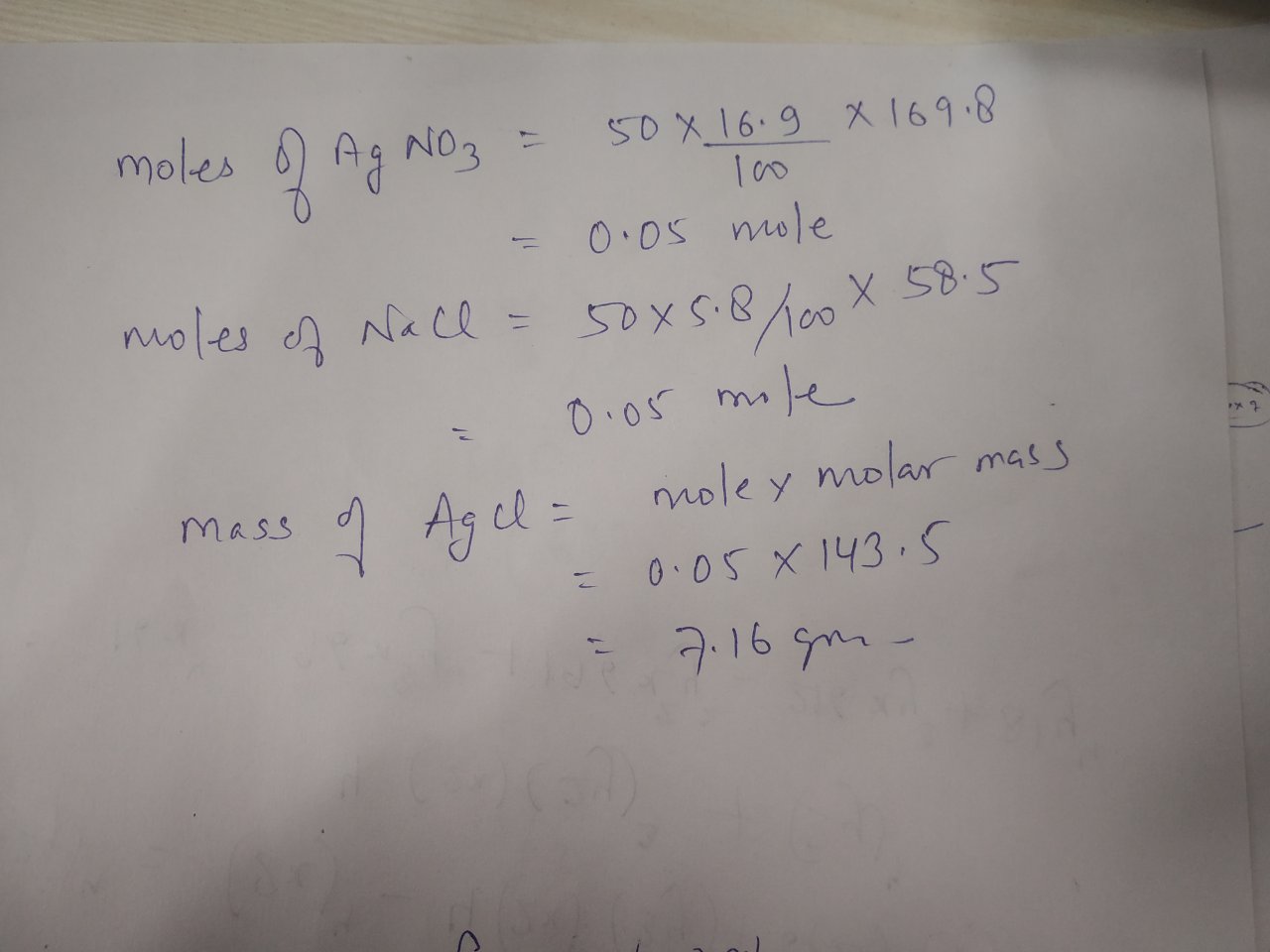
Your idea is magnificent
I consider, that you are not right. I am assured. I can defend the position. Write to me in PM, we will talk.
It is difficult to tell.