What are isotopes and isobars give examples
The atoms of an element with the same atomic number but different atomic masses are termed isotopes.
Isobar are elements that differ in chemical properties but have the same physical property. So, we can say that isobars are those elements that have a different atomic number but the same mass number. In contrast, Isotopes are those elements having the same atomic number and different mass numbers. Isobars are atoms nuclides of different chemical elements which differs in the chemical property but has the same physical property. So, we can say that isobars are those elements which have a different atomic number but the same mass number. Their chemical property is different because there is a difference in the number of electrons.
What are isotopes and isobars give examples
Isobars are a group of elements that have the same mass number but different atomic numbers. In an isobar, we have different numbers of protons but the same number of nucleons, i. An example of isobar is carbon and nitrogen as they both have 14 nucleons in their nucleus but different atomic numbers, the atomic number of carbon is 6 and the atomic number of nitrogen is 7. The isobar has somewhat the same physical properties but different chemical properties. In this article, we will learn about isobars, their examples, their differences with isotopes and others in detail. Isobars are a group of elements from the periodic table that have different atomic numbers but their mass number are the same. We can say that in isobars the number of protons in their nucleus is different but the sum of the number of protons and neutrons is the same. For example, Argon 18 Ar 40 , Potassium 19 K 40 , and Calcium 20 Ca 40 are isobars as they all have 40 as their mass number but their atomic number are different. This happens because they have different atomic numbers but the sum of protons and neutrons in their nucleus is different. The table added below shows the following condition,. There are various examples in the periodic table that are isobars, i. Various examples of the isobars are discussed below,. Sodium 24 and Magnesium 24 are the isobars of each other and we can represent their condition as,. Aluminium 27 and Silicon 27 are the isobars of each other and we can represent their condition as,.
As we all know, every atom is made of electrons, protons, and neutrons. Share your thoughts in the comments.
Isobar is an element that differs in chemical properties, but it has similar physical properties. Hence, we can say that isobars are elements that have a different atomic number but the same mass number. Also, they have a different chemical property because there is a difference in the electron count. An isobar contains the same atomic mass but a different atomic number because an added number of neutrons recompense the number of nucleons. An example of two isotopes and isobars is nickel and iron.
The name was given by Alfred Walter Stewart in It is originally taken from the combination of Greek words- isos means equal and bar means weight. Atoms of chemical elements having same atomic mass but a different atomic number are called Isobars. The sum of the number of protons and neutrons together form the atomic mass. Therefore, we can also say the number of nucleons present in the nucleus is equal to the atomic mass of an atom.
What are isotopes and isobars give examples
Isobar are elements that differ in chemical properties but have the same physical property. So, we can say that isobars are those elements that have a different atomic number but the same mass number. In contrast, Isotopes are those elements having the same atomic number and different mass numbers. Isobars are atoms nuclides of different chemical elements which differs in the chemical property but has the same physical property. So, we can say that isobars are those elements which have a different atomic number but the same mass number. Their chemical property is different because there is a difference in the number of electrons. It has the same atomic mass but different atomic no. This is because an additional number of neutrons compensates for the difference in the number of nucleons. The example of two Isotopes and Isobars is iron and nickel. Both have the same mass number which is 58 whereas the atomic number of iron is 26, and the atomic number of nickel is
5 megabytes in bytes
Difference between Electrovalency and Covalency. Electronic Configuration Of All Elements. View Test Series. The number of protons is always the same in an element, but the number of neutrons keeps on changing. Enhance the article with your expertise. Calculus Cheat Sheet. In an isobar, we have different numbers of protons but the same number of nucleons, i. Both have the same mass number which is 58 whereas the atomic number of iron is 26, and the atomic number of nickel is What are Isotopes? Explore offer now.
Isobar is an element that differs in chemical properties, but it has similar physical properties. Hence, we can say that isobars are elements that have a different atomic number but the same mass number. Also, they have a different chemical property because there is a difference in the electron count.
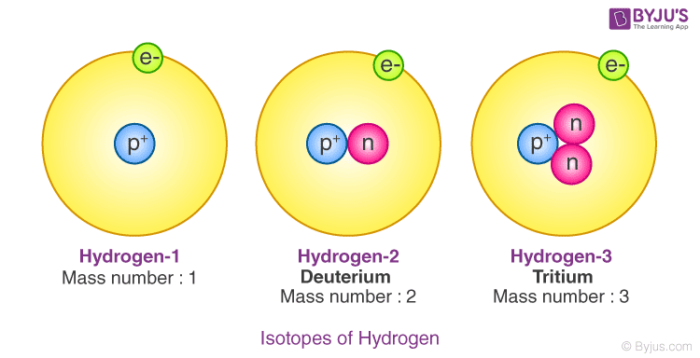
Alpha, beta, and gamma rays are emitted by unstable isotopes. Get the app right away to take advantage of some exclusive deals waiting for you! They have the same mass number. Isobars are atoms nuclides of different chemical elements which differs in the chemical property but has the same physical property. What is Fractional Atomic Mass? You can relate the concepts of isotopes with this example. This Chemistry article focuses on the meaning, differences, examples, and uses of Isotopes and Isobars. In general, there are different physical properties. Let us know something about the isotopes of hydrogen: There are three isotopes of hydrogen and these are protium, deuterium, and tritium. They have the same number of protons but a different number of neutrons. Element No.

Absolutely with you it agree. In it something is also idea excellent, agree with you.
I confirm. So happens. We can communicate on this theme. Here or in PM.
I think it already was discussed, use search in a forum.