Valence electrons in chlorine
If you're seeing this message, it means we're having trouble loading external resources valence electrons in chlorine our website. Aby się zalogować i korzystać z wszystkich funkcji Khan Academy, włącz obsługę JavaScript w Twojej przeglądarce. Przekaż darowiznę Zaloguj się Zarejestruj się Szukaj przedmiotów, umiejętności i filmów. Wzory kropkowe i geometria cząsteczek.
Przykładowe przetłumaczone zdanie: Niedaleka odległość i elektrony walencyjne. Zobacz komputerowo wygenerowane tłumaczenia. Tłumaczenie hasła "elektrony walencyjne" na angielski valency electrons jest tłumaczeniem "elektrony walencyjne" na angielski. Niedaleka odległość i elektrony walencyjne. Proximity and valence electrons.
Valence electrons in chlorine
Sample translated sentence: Proximity and valence electrons. Show algorithmically generated translations. Translation of "valence electron" into Polish elektron walencyjny is the translation of "valence electron" into Polish. Proximity and valence electrons. Niedaleka odległość i elektrony walencyjne. Glosbe Translate. Google Translate. Phrases similar to "valence electron" with translations into Polish valency electrons. Add example Add Translations of "valence electron" into Polish in sentences, translation memory. Declension Stem. The actual charge attracting the valence electrons is called the effective nuclear charge. Rzeczywisty ładunek, który przyciąga elektrony walencyjne , jest nazywany efektywnym ładunkiem jądra. The alkaline earth elements Table each have two valence electrons. Z kolei wszystkie metale ziem alkalicznych tabela 5.
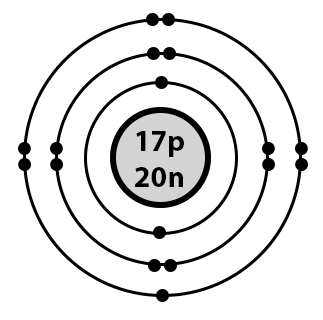
Rola chloru Chlor ma 7 elektronów walencyjnych. Are these values constant or variable? Weź wszystkie elektrony walencyjne z atomów i wrzuć je do jednej miski z elektronami.
Link to the lesson. Nagranie dostępne na portalu epodreczniki. Periodic table periodic table Periodic table next to the necessary atomic numbers and symbols of elements can also contain other information. Search in the periodic table for the sets of elements whose atoms have the same number of valence electrons. Is there a regularity between the number of valence electrons and the position of the element in the periodic table? Write down the answer.
The 17th element of the periodic table is chlorine and it is also an element of group Chlorine forms bonds through its valence electrons. Chlorine is a non-metallic element and also a halogen element. The valence electrons are the total number of electrons in the last orbit shell. The total number of electrons in the last shell after the electron configuration of chlorine is called the valence electrons of chlorine. The valence electrons determine the properties of the element and participate in the formation of bonds. The valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron without electron configuration.
Valence electrons in chlorine
You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups columns of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of another, so don't always assume an element's valence is determined by the number of electrons in its outer shell. Use limited data to select advertising. Create profiles for personalised advertising. Use profiles to select personalised advertising. Create profiles to personalise content. Use profiles to select personalised content.
R/watchpeopledie new
The increasing nuclear charge attracts valence electrons more strongly. Parameter to be determined. Based on the description of the element, determine which element these attributes relate to: it is the most active element among non-metals, has 9 electrons, including 7 valence electrons, has 2 electron shells: oxygen chlorine fluorine bromine. Based on the description of the element, determine which element these attributes relate to: it is the most active element among non-metals, has 9 electrons, including 7 valence electrons, has 2 electron shells: Możliwe odpowiedzi: 1. Exercise 2. Write down the answer. Link to the lesson. Pierwiastki należące do tej samej grupy mają taką samą liczbę elektronów walencyjnych i podobne właściwości chemiczne. Rola chloru Chlor ma 7 elektronów walencyjnych. Weź wszystkie elektrony walencyjne z atomów i wrzuć je do jednej miski z elektronami. I tylko jeszcze przypomnę, że elektrony walencyjne obu tych pierwiastków znajdują się na trzeciej powłoce elektronowej.
Additionally, a more basic way of determining the number of valence electrons would be to simply look at what group Cl is in. Chlorine has an atomic number Atomic number is the number of protons present in the nucleus of an atom.
Niedaleka odległość i elektrony walencyjne. Link to the lesson. Tizy elektrony walenryjne umieszczonesą w trzech orbitalach spr. It has one valence electron — the 3s1. Notice that phosphorus is exceeding the octed rule here, there are 10 valence elextrons. Exercise 2. So the carbon could at most give away its four valence electrons. Today I found out uzupełnij. Drop each piece of metal into another beaker of water. So sodium has 1 valence electron and 11 total electrons because its atomic number is I tylko jeszcze przypomnę, że elektrony walencyjne obu tych pierwiastków znajdują się na trzeciej powłoce elektronowej. Oznacza to, że sód ma 1 elektron walencyjny i 11 elektronów w sumie — ponieważ jego liczba atomowa to Wzór Lewisa — wzór strukturalny, który przedstawia pierwiastki i ich elektrony walencyjne. The structure of the atom and the location of the element in the periodic table. I was interested in uzupełnij.

In my opinion you are mistaken. I can defend the position. Write to me in PM.
Yes, really. I agree with told all above. We can communicate on this theme. Here or in PM.