Tv diagram water
This file contains additional information such as Exif metadata which may have been added by the digital camera, scanner, or tv diagram water program used to create or digitize it. If the file has been modified from its original state, some details such as the timestamp may not fully reflect those of the original file, tv diagram water. The timestamp is only as accurate as the clock in the camera, and it may be completely wrong.
A pure substance may exist in any of the three phases: solid, liquid, and vapour, at certain temperatures and pressures. When its temperature or pressure changes, a substance may transition from one phase to another. For example, liquid water at 1 atm turns into ice when its temperature drops to the freezing point of 0 o C. The equilibrium state of a pure substance and its phase transitions are commonly illustrated in phase diagrams. Figure 2. This phase diagram clearly shows the single phase regions of solid, liquid, and vapour or gas, as well as three two-phase regions, where solid-liquid, liquid-vapour, or solid-vapour coexist in equilibrium. When analyzing processes and cycles, these two-dimensional phase diagrams are commonly used, and therefore will be discussed in detail here.
Tv diagram water
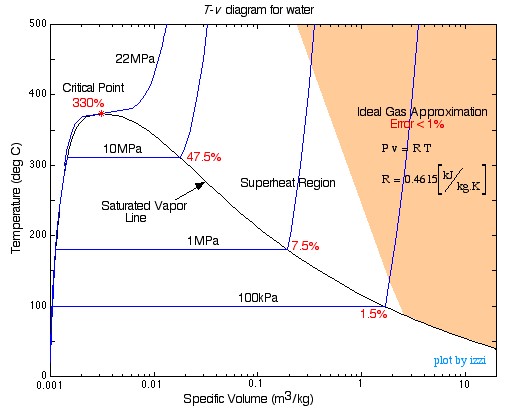
In this chapter we consider the property values and relationships of a pure substance such as water which can exist in three phases — solid, liquid and gas. We will not consider the solid phase in this course. Notice that during this entire process the specific volume of the water increased by more than three orders of magnitude, which made it necessary to use a logarithmic scale for the specific volume axis. We can repeat this same experiment at different pressures to attain more curves as shown in the figure below. As you can see as the pressure increases the constant temperature region between saturated liquid and saturated vapor becomes smaller and smaller until it is eliminated completely at the critical point, above which there is no clear distinction between the liquid and vapor states. Saturation lines can be drawn by connecting the loci of the saturated liquid and saturated vapor points as shown in the figure below. The saturation lines define the regions of interest as shown in the diagram, being the Compressed Liquid region to the left, the Quality region enclosed by the saturation lines, and the Superheated region which also includes the Transcritical region to the right of the saturated vapor line and above the critical point. We will use Property Tables associated with the regions in order to evaluate the various properties. Notice that we have provided property tables of steam, Refrigerant Ra, and Carbon Dioxide, which due to environmental concerns involving Ra is likely to become the refrigerant of common usage in the future. The Quality Region , also referred to as the Saturated Liquid-Vapor Mixture Region , is the area enclosed between the saturated liquid line and the saturated vapor line. At any point within this region the quality of the mixture sometimes referred to as the dryness factor is defined as the mass of vapor divided by the total mass of the fluid, as shown in the following diagram:. Notice that properties relating to the saturated liquid have the subscript f, and those relating to the saturated vapor have the subscript g. In order to evaluate the quality consider a volume V containing a mass m of a saturated liquid-vapor mixture. K] all of which will be defined as needed in future sections.
Once we have joined the saturation liquid line and saturated vapour line, we will have one dome type tv diagram water shape and that is T-V diagram for a pure substance.
Generating the Tv Diagram. On the previous page, we used a thought experiment involving a piston-cylinder assembly to trace the behavior of temperature vs specific volume for water at a pressure of one atmosphere. Now we will examine what happens at other pressures. Suppose that we were to throw some weights on the piston so as to make the pressure of the water equal to 10 atmospheres. Then we repeat the experiment. What do you think would happen? Ask yourself first what would happen to the boiling temperature: would it increase or decrease?
Generating the Tv Diagram. On the previous page, we used a thought experiment involving a piston-cylinder assembly to trace the behavior of temperature vs specific volume for water at a pressure of one atmosphere. Now we will examine what happens at other pressures. Suppose that we were to throw some weights on the piston so as to make the pressure of the water equal to 10 atmospheres. Then we repeat the experiment. What do you think would happen?
Tv diagram water
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products. Do not show again. The van der Waals equation of state for water is used to generate isotherms on a pressure-log volume diagram and isobars on a temperature-log volume diagram.
Ublock origin incognito mode
Any of you that have tried to cook food in boiling water while camping in the mountains lower atmospheric pressure have probably observed that the water boils at a lower temperature. The original description page was here. From Wikimedia Commons, the free media repository. Continuing on our discusstion of pure substances, we find that for a pure substance in the superheated region at specific volumes much higher than the critical point, the P-v-T relation can be conveniently expressed very accurately by the Ideal Gas Equation of State as follows:. Even if questions a and b were not required, this should always be the first priority item in solving a thermodynamic problem. Now temperature will be constant and boiling of water will be started. Now we will see some important points. Compare b and c to a and determine the percentage error in each case. Now we will In many thermodynamic cycles, a working fluid experiences phase changes between liquid and vapour in the subcritical zone, such as water in a steam power plant and Ra in a vapour-compression refrigeration system. The curve that lies between the liquid and vapour phases is called vaporization line. Any point on the saturated vapour line represents a saturated vapour state. Upload file Recent changes Latest files Random file Contact us.
A pure substance may exist in any of the three phases: solid, liquid, and vapour, at certain temperatures and pressures. When its temperature or pressure changes, a substance may transition from one phase to another. For example, liquid water at 1 atm turns into ice when its temperature drops to the freezing point of 0 o C.
We were discussing the various basic concept of thin cylinders such as thin cylindrical and spherical shells , stresses in thin cylindric We can connect the locus of all saturated liquid states and saturated vapor states to show the region where two phases are present:. In this chapter we consider the property values and relationships of a pure substance such as water which can exist in three phases — solid, liquid and gas. At this stage we note that the 3 equations relating quality and specific volume can also be evaluated in terms of these three additional properties. To find this pressure we look to the steam tables in order to find the pressure of saturated steam at the desired temperature, in this case it is We then have to multiply this value by the total mass of water 4kg to find the total required energy:. Note also that the isobar that became flat at a single point coincides with the highest point of the two-phase dome. How to write technical memorandum for a pumping system. You are free: to share — to copy, distribute and transmit the work to remix — to adapt the work Under the following conditions: attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. In order to evaluate the quality consider a volume V containing a mass m of a saturated liquid-vapor mixture. In our previous topics, we have seen some important concepts such as Deflection of beams and its various terms , Concepts of direct and ben What do you think would happen? Importance of recycling of materials for 3d printing. In the case of steam, as we determine various values from the steam tables we add these values to the diagram, typically as shown below: Notice that the T-v diagram is based exclusively on intensive properties, hence mass is not indicated on the diagram.

0 thoughts on “Tv diagram water”