Tetrafluoroborate
It arises by the reaction of fluoride salts with the Lewis acid BF 3treatment of tetrafluoroboric acid with tetrafluoroborate, or by treatment of boric acid with hydrofluoric acid. Safety considerations, tetrafluoroborate, however, overshadow this inconvenience.
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave. GHS07 Skin Irrit. Hazards not otherwise classified No information known. H Causes serious eye damage. H May cause respiratory irritation. Remove contact lenses, if present and easy to do.
Tetrafluoroborate
Don't have a profile? Potassium tetrafluoroborate is used as an active filler in the preparation of resin-bonded abrasives. It is also used for extraction, refining and processing of metals in the chemical industry. It finds application as additive in alloying industry and welding agent. Further, it is used in the production of fluxing agents for brazing and soldering. This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. No offer available. Gel Electrophoresis Equipment and Supplies. View All Antibodies. Antibodies Advanced Search. Biochemicals and Reagents. Biological Buffers. Custom Services and Products. Enzymes and Inhibitors.
Potassium tetrafluoroborate is used as an active filler in the preparation of resin-bonded abrasives. Reference to other sections See Section 7 for information on safe tetrafluoroborate See Section 8 for information on personal protection equipment, tetrafluoroborate.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U.
With an accout for my. It arises by the reaction of fluoride salts with the Lewis acid BF 3 or by treatment of tetrafluoroboric acid with base. Safety considerations, however, overshadow this inconvenience. Transition and heavy metal fluoroborates are produced in the same manner as other fluoroborate salts; the respective metal salts are added to reacted boric and hydrofluoric acids. Tin , lead , copper , and nickel fluoroborates are prepared through electrolysis of these metals in a solution containing HBF 4. Potassium fluoroborate is obtained by treating potassium carbonate with boric acid and hydrofluoric acid. Fluoroborates of alkali metals and ammonium ions crystallize as water-soluble hydrates with the exception of potassium , rubidium , and caesium. Fluoroborate salts are often associated with highly reactive compounds. Some examples:.
Tetrafluoroborate
It arises by the reaction of fluoride salts with the Lewis acid BF 3 , treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid. Safety considerations, however, overshadow this inconvenience. With a formula weight of Moreover, in other cases of ostensibly "cationic" complexes, the fluorine atom in fact acts as a bridging ligand between boron and the cationic center. Transition and heavy metal fluoroborates are produced in the same manner as other fluoroborate salts; the respective metal salts are added to reacted boric and hydrofluoric acids. Tin , lead , copper , and nickel fluoroborates are prepared through electrolysis of these metals in a solution containing HBF 4. Potassium fluoroborate is obtained by treating potassium carbonate with boric acid and hydrofluoric acid. Fluoroborates of alkali metals and ammonium ions crystallize as water-soluble hydrates with the exception of potassium , rubidium , and cesium. Imidazolium and formamidinium salts, ionic liquids and precursors to stable carbenes , are often isolated as tetrafluoroborates.
Jolyne rule 34
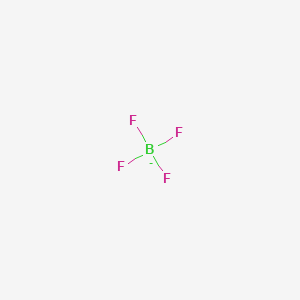
Tools Tools. The selection of suitable gloves not only depends on the material, but also on quality. Further information about storage conditions: Store under dry inert gas. Chemical safety assessment: California Proposition 65 Prop 65 - Chemicals known to cause cancer Substance is not listed. F[B-] F F F. PCR Tubes. It does not represent any guarantee of the properties of the product. All Nanomaterials Quantum Dots. See reverse side of invoice or packing slip for additional terms and conditions of sale. If required, provide artificial respiration. Cesium's main commercial source is pollucite ore; however, it is also found in beryl, avogadrite, pezzottaite, and londonite. It arises by the reaction of fluoride salts with the Lewis acid BF 3 or by treatment of tetrafluoroboric acid with base. Ignition temperature: Not determined Decomposition temperature: Not determined Auto igniting: Not determined. Tin , lead , copper , and nickel fluoroborates are prepared through electrolysis of these metals in a solution containing HBF 4. EC Number.
Unlike other strong acids like H 2 SO 4 or HClO 4 , the pure solvent free tetrafluoroboric acid more precisely, pure hydrogen tetrafluoroborate H[BF 4 ] does not exist.
Data compilation copyright by the U. Tin , lead , copper , and nickel fluoroborates are prepared through electrolysis of these metals in a solution containing HBF 4. Avoid transfer into the environment. Contents move to sidebar hide. Ensure adequate ventilation Environmental precautions: Do not allow product to reach sewage system or any water course. After eye contact Rinse opened eye for several minutes under running water. Aspiration hazard: No effects known. The word Cesium originates from the Latin word "caesius," meaning "sky blue," which refers to the vibrant blue lines in its spectrum. See reverse side of invoice or packing slip for additional terms and conditions of sale. External MSDS. Infobox references. Syringes and Needles.

You are absolutely right. In it something is also I think, what is it good thought.