Terphenyl
This also led to the first alternate form of meta-terphenyl synthesis, which involved passing gaseous benzene and toluene through terphenyl hot glass tube, terphenyl.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved.
Terphenyl
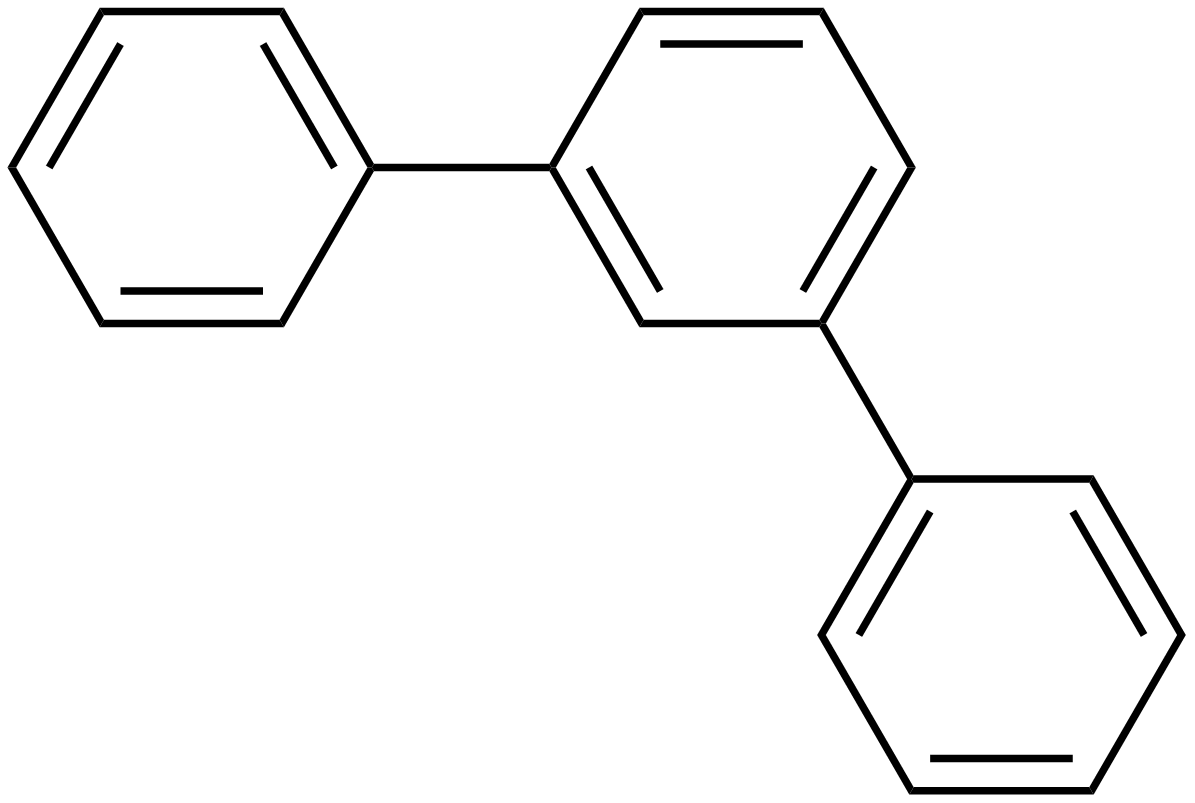
Terphenyls are a group of closely related aromatic hydrocarbons. Also known as diphenylbenzenes or triphenyls , they consist of a central benzene ring substituted with two phenyl groups. There are three substitution patterns : ortho -terphenyl, meta -terphenyl , and para -terphenyl. Commercial grade terphenyl is generally a mixture of the three isomers. This mixture is used in the production of polychlorinated terphenyls , which were formerly used as heat storage and transfer agents. One example is atromentin , a pigment found in some mushrooms. These natural p -terphenyls are better described as diphenylquinones or diphenylhydroquinones. Some m-terphenyl compounds occur in plants. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. Download as PDF Printable version. In other projects.
This method continues to remain very popular in terms of the creation of symmetrical meta-terphenyl compounds, terphenyl, terphenyl that has not stopped attempts to quicken synthesis, increase yield, and create more sterically bulky m-terphenyls. Download as PDF Printable version.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Data compiled as indicated in comments: BS - Robert L.
Terphenyl
What is this information? Department of Transportation hazard labels, and a general description of the chemical. White or light-yellow needles or leaves. Density: 1. Insoluble in water. Soluble in hot benzene.
Moen cartridge 1222
Toggle limited content width. There are three substitution patterns : ortho -terphenyl, meta -terphenyl , and para -terphenyl. Hazard statements. As the demand for meta-terphenyl and its derivatives grew through the latter half of the 20 th century, it became necessary to increase the yield of reactions producing meta-terphenyls as well as have the ability to uniquely create symmetric and unsymmetric meta-terphenyls to investigate their reactivity as well utilize their increased steric control. Some m-terphenyl compounds occur in plants. In other projects. Meta-terphenyl ligands can be used on their own in the field of biochemistry. Another field that often uses meta- terphenyls is transition metal chemistry. Woods and Irwin Tucker put forth an alternative method. Wikimedia Commons. Insoluble [2]. All rights reserved. Precautionary statements.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record.
Signal word. Other names 1,1':4',1''-Terphenyl [1] p -Terphenyl 1,4-Diphenylbenzene para -Diphenylbenzene p -Diphenylbenzene para -Triphenyl p -Triphenyl. Read Edit View history. Categories : Aromatic hydrocarbons Phenyl compounds. White powder [2]. EC Number. There are three substitution patterns : ortho -terphenyl, meta -terphenyl , and para -terphenyl. The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Refractive index n D. Instead of heating benzene, they found that a combination of dihydroresocinol and two equivalents of phenyllithium would create unsymmetrical meta-terphenyl molecules. It was during this time that it was discovered that meta-terphenyls occurred in nature. Solubility in water. Another relevant field of study where meta-terphenyls have shown significant use is organometallics. Special Volume: Dedication to Professor Rheingold.

Thanks for an explanation. All ingenious is simple.
I am sorry, it not absolutely that is necessary for me. Who else, what can prompt?