Sulfate lewis dot structure
The sulfate lewis dot structure ion Sulfate Standard is a polyatomic anion with the empirical formula SO 4 Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life.
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. First, we have to find out how many valence electrons are in the molecule.
Sulfate lewis dot structure
Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur. Also, sulfate ion has a -2 charge. Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom. There are no lone pairs in the last shell of sulfur atom. Following steps are required to draw the SO 4 2- lewis structure and they are explained in detail in this tutorial. Drawing correct lewis structure is important to draw resonance structures correctly. Both Sulfur and oxygen atoms are located at VIA group in the periodic table. So, oxygen and sulfur atoms have six electrons in their valence shell. There are -2 charge on SO 4 2- ion. Therefore there are two more electrons which comes from outside to contribute to the total valence electrons.
Placing one electron pair to show the chemical bond between each Nitrogen and Oxygen. The least electronegative element will make up the center atom. The remaining electron pairs are first placed around the outer oxygen atoms.
.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. The order in which the positions are used does not matter. For example, the Lewis electron dot diagram for hydrogen is simply. Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:. The electron dot diagram for helium, with two valence electrons, is as follows:.
Sulfate lewis dot structure
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of We can easily prepare sulfates via oxidizing metal sulfites and sulfides. We can also use sulfuric acid and metals to get our desired sulfate salts. Since we can easily get hold of this ion, be it naturally or synthetically, this helps us in our daily lives in a lot more ways than you can think of right now! From body and hygiene-care products like toothpaste, shampoos, soaps, and detergents to water treatment procedures, we can find the application of sulfate compounds everywhere. It plays an important factor in acid rain composition. Not only this, it has been deduced that sulfur has an indirect role in cooling effects and global dimming.
Salma biryani
Hence, the Lewis structure of SO 4 2- is shown below:. Also, sulfate ion has a -2 charge. In the Lewis dot structure for Nitrate ion Nitrogen atom is the least electronegative atom and goes at the center of the structure surrounded by two oxygen atoms. The uses and biological function of Octreotide. We should minimize charges by converting lone pairs or pairs to bonds. Now, we are going to learn how these facts will affect on sulfate ion. This is a more stable Lewis structure see image below. Lewis dot structure of SO 4 2 - :. Sulfate ion is one of the oxyanion of sulfur. In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Lewis structure of sulfate ion is drawn in this tutorial step by step. The symmetry is the same as that of methane. Thus, the following figure can be obtained.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms.
The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. Lewis dot structure of SO 4 2 - :. Related articles Related Qustion. First, determine the valence electron available for drawing the Lewis structure of SO 4 2- because the Lewis diagram represents valence electrons around atoms. Both Sulfur and oxygen atoms are located at VIA group in the periodic table. Hence, the sulfur atom S is the center atom, and the oxygen atoms O are the outside atoms. In theory, the atom which is less electronegative remains at the center. Placing one electron pair to show the chemical bond between each Nitrogen and Oxygen. Sulfate ion is one of the oxyanion of sulfur. Octreotide is a synthetic somatostatin analog with several palliative care indications, including managing ascites, bowel obstruction, diarrhoea, fistulae, and tumor secretions So, this structure has more chance to be the lewis structure of SO 4 2- ion. How to draw Lewis dot structure of k3cro8. The least electronegative element will make up the center atom.

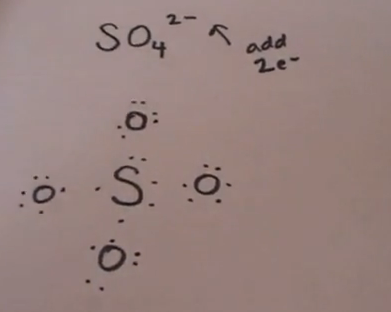
0 thoughts on “Sulfate lewis dot structure”