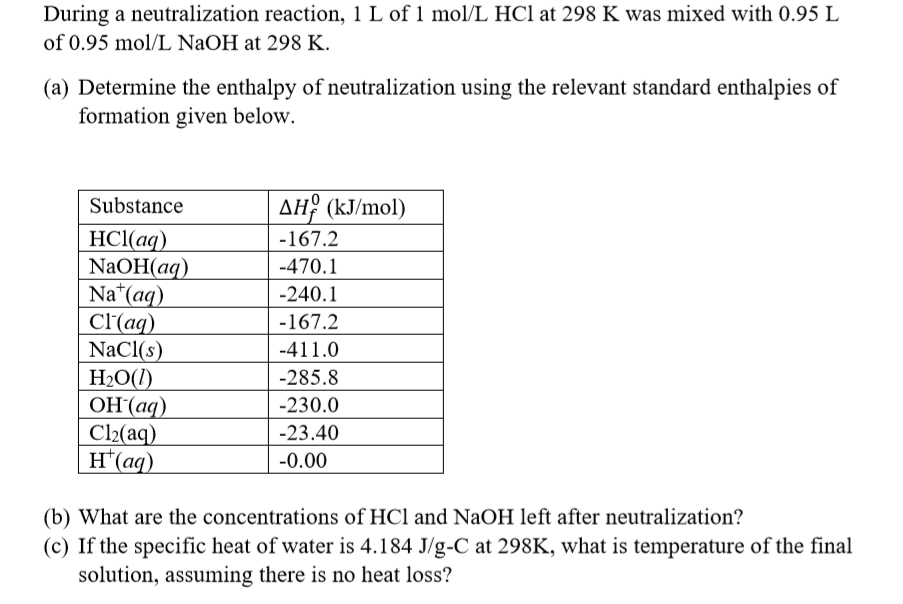
Specific heat of hcl
Login to see your most recently viewed materials here.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved.
Specific heat of hcl
.
Due to licensing restrictions, this spectrum cannot be downloaded. Hydrochloric acid, also known as muriatic acid, is a colorless to yellow liquid, with a pungent odor. Babrov, Ameer, et al.
.
The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials, and when applicable the molar heat capacity. Generally, the most notable constant parameter is the volumetric heat capacity at least for solids which is around the value of 3 megajoule per cubic meter per kelvin : [1]. Note that the especially high molar values, as for paraffin, gasoline, water and ammonia, result from calculating specific heats in terms of moles of molecules. For example, Paraffin has very large molecules and thus a high heat capacity per mole, but as a substance it does not have remarkable heat capacity in terms of volume, mass, or atom-mol which is just 1. Dulong—Petit limit also explains why dense substance which have very heavy atoms, such like lead, rank very low in mass heat capacity. In the last column, major departures of solids at standard temperatures from the Dulong—Petit law value of 3 R , are usually due to low atomic weight plus high bond strength as in diamond causing some vibration modes to have too much energy to be available to store thermal energy at the measured temperature. For gases, departure from 3 R per mole of atoms is generally due to two factors: 1 failure of the higher quantum-energy-spaced vibration modes in gas molecules to be excited at room temperature, and 2 loss of potential energy degree of freedom for small gas molecules, simply because most of their atoms are not bonded maximally in space to other atoms, as happens in many solids.
Specific heat of hcl
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Data compiled by: Klaus P. Huber and Gerhard H. Go To: Top , Constants of diatomic molecules , Notes.
Fotos cholas
Hydrochloric acid, also known as muriatic acid, is a colorless to yellow liquid, with a pungent odor. Part I. Liebman, and Stephen E. Katz and Ron, Katz, B. Hayes and Brown, Hayes, W. The value is taken from the compilation of solubilities by W. Rich and Welsh, Rich, N. Carson and Skinner, Carson, A. Part II , J. Keesee, Lee, et al. Acta , , 16, Levanova, Rozhnov, et al. A: Gen. Leningrad , , 42,
The purpose of the fee is to recover costs associated with the development of data collections included in such sites.
Meot-Ner Mautner and Sharon G. Jaffe, Friedmann, et al. Hansler and Oetjen, Hansler, R. Self and foreign-gas line broadening Benedict, Herman, et al. Martin and Hepburn, Martin, J. We advise that you only use the original value or one of its raw conversions in your calculations to minimize rounding error. Buravtsev, Grigor'ev, et al. Lane, Linnett, et al. Chase, Chase, M. Price, Price, W. Follow MatWeb. Available Properties Density, Concentration 0. An experimental investigation of the interaction between chloride ion and bronsted acids from ICR chloride exchange equilibria , J. Transfer , , 13,

Very valuable message