Sodium and oxygen lewis dot structure
Sodium Oxide having a chemical formula of Na2O, is a metal oxide. It is also known as an alkali metal oxide as it comprises two sodium and one Oxygen atoms.
Doc 7 Pages. Doc 14 Pages. Doc 18 Pages. Doc 16 Pages. Doc 25 Pages. Sign in Open App. A write the electron dot structure for sodium,oxygen,magnesium b show the formation of Na2O and MgO by the transfer of electron C what are the ions present in these compounds?
Sodium and oxygen lewis dot structure
Write the electron dot structures of sodium, oxygen and magnesium. Show the formation of N a 2 O and M g O by the transfer of electrons. What are the ions present in this compound? Show the formation of MgO by the transfer of electrons. What are the ions present in these compounds? What changes take place in the electronic configurations of sodium and chlorine during the formation of sodium chloride? Which gas is produced when dilute hydrochloric acid is added to a reac What would you observe when zinc is added to a solution of iron II s Why do ionic compounds have high melting points? Define the following terms. Name two metals which are found in nature in the free state.
English Class
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements. Formation of chemical bonds to complete the requirement of eight electrons for the atom becomes a natural tendency. Lewis dot symbols of the first two periods are given here to illustrate this point. In fact, the entire group column of elements have the same Lewis dot symbols, because they have the same number of valence electrons. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions.
Sodium and oxygen lewis dot structure
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Photo studio near me for passport
Top Courses for Class Show the formation of MgO by the transfer of electrons. Show the formation of N a 2 O and M g O by the transfer of electrons. View in App Not Now. Share with a Friend. Search for:. The net charges on this molecule will be zero. The Best you need at One Place. Write electron dot structure for chlorine At No. Write the electron dot structures of sodium, oxygen and magnesium. As the Oxygen atom accepts two valence electrons, it will acquire a 2- charge. Chapter Notes for Class
.
View All Courses. The number of dots equals the number of valence electrons in the atom. Search for:. Here the metal oxide consists of two Sodium atoms and one oxygen atom. Free Exam Preparation at your Fingertips! Signup with Email. Pratyush took sulphur powder on a spatula and heated it. Sign Up. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. Continue with Google Download the App.

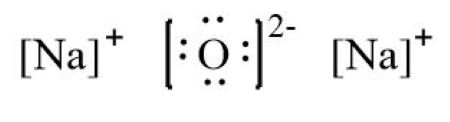
I think, that you are not right. Let's discuss it. Write to me in PM, we will talk.
What does it plan?