So3 lewis diagram
Sulfur trioxide molecule contains one sulfur atom and three oxygen atoms. We will construct the lewis structure of SO 3 molecule by following VSEPR theory rules and considering stability of intermediate structures, so3 lewis diagram. Finally, after obtaining the lewis structure of SO 3we can determine the hybridization of atoms.
Sulfur trioxide is a compound with the chemical formula SO 3. This compound is widely postulated as the active sulfonating agent in electrophilic aromatic substitutions. Sulfur trioxide is a crucial compound for atmospheric sulfuric acid H 2 SO 4 formation, acid rain formation, and other atmospheric physicochemical processes. However, the sources of SO 3 during the early morning and night, when OH radicals are scarce, still need to be fully understood. Sulfur trioxide is not only environmentally harmful but also highly corrosive and poses a significant threat to the safe operation of coal-fired power plants[].
So3 lewis diagram
Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents Lewis Electron Dot Structures. Molecular Orbital Theory and ab initio calculations can be used to calculate and draw the electrostatic potential ESP of a molecule. The molecular electrostatic potential is the potential energy of a proton at a particular location near a molecule. There are negative and positive ESP's. Negative electrostatic potential corresponds to a attraction of the proton by the concentrated electron density in the molecules mainly from lone pairs, pi-bonds, Red color in electrostatic potential drawings show areas with high electron density. Positive electrostatic potential corresponds to repulsion of the proton by the atomic nuclei in regions where low electron density exists and the nuclear charge is incompletely shielded colored blue. Blue color in electrostatic potential drawings show areas with low electron density. Several ab initio softwares calculate electrostatic potentials based on quantum mechanically derived molecular orbitals. The electrostatic potential of SO 3 derived by ab initio calculations is shown below:. It is to be noted that most of the negative charge electron rich areas, colored red is concentrated on the oxygen atoms of SO 3 as it is expected from the Lewis structures of SO 3 and by chemical intuition. Most of the positive charge electron deficient areas, colored blue is concentrated on the S atom as it is expected from the Lewis structures of SO 3 and by chemical intuition. Lewis Dot Structure of the sulfite ion SO 3
How to draw the Lewis structure of CO. But because these three bonds are conjugated, they are actually completely equivalent.
.
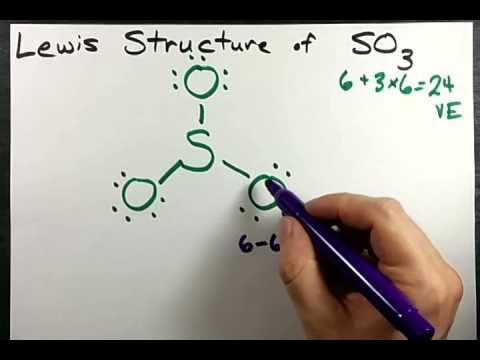
The SO 3 Lewis structure illustrates how the atoms of sulfur trioxide, a molecule composed of one sulfur atom and three oxygen atoms, are arranged. Within the SO 3 Lewis structure, the sulfur atom is bonded to three oxygen atoms through double bonds. Additionally, each oxygen atom has two lone pairs of electrons associated with it. To draw this structure, begin by sketching a rough diagram of the molecular arrangement. Next, indicate the lone pairs on each atom and check for any formal charges. If formal charges are present, convert lone pairs to minimize these charges. Repeat this process until all charges are minimized.
So3 lewis diagram
SO 3 stands for Sulfur Trioxide. This is one of the most pollutant chemical compounds in the gaseous form. It is also a primary agent in the acid rain. The main use of this component is to make sulfuric acid for industrial purposes. SO 3 which is also spelled as Sulphur Trioxide sometimes, is a trigonal planar molecule that is non-flammable. In this article, I will provide you some information regarding SO 3 molecular geometry with the explanations of Lewis structure, polarity, and hybridization. Being an intelligent and well-practiced human being, you must know what is molecular geometry , but let me revise it for the all young students out there. Molecular geometry is the three-dimensional structure of the atoms which helps in the constitution of a molecule.
Hair splinter español
Sulfur trioxide SO 3 Sulfur trioxide is a oxide of sulfur and colourless inorganic gas. Sulfur trioxide gas is produced due to oxidation of sulfur dioxide gas in air. Adenosine is a ubiquitous extracellular signaling molecule and plays a fundamental role in the regulation of coronary microcirculation through activation of adenosine receptors ARs This is due to the trigonal planar molecular geometry, where the three oxygen atoms are arranged symmetrically around the central sulfur atom. Related articles Related Qustion. Theoretically, the Lewis structure of SO3 is shown in the figure B above. Several ab initio softwares calculate electrostatic potentials based on quantum mechanically derived molecular orbitals. Below, That step are done. Sulfur trioxide We will construct the lewis structure of SO 3 molecule by following VSEPR theory rules and considering stability of intermediate structures. However, the sources of SO 3 during the early morning and night, when OH radicals are scarce, still need to be fully understood. Lewis Dot Structure of the sulfite ion SO 3
Sulfur trioxide is a compound with the chemical formula SO3. This compound is of great importance and studied widely as it reacts with the water present in the air to produce sulfuric acid. When this sulfuric acid, in the gaseous state, mixes with the rain and falls on the Earth, it is called acid rain.
The electrostatic potential of SO 3 derived by ab initio calculations is shown below:. We will construct the lewis structure of SO 3 molecule by following VSEPR theory rules and considering stability of intermediate structures. This is due to the trigonal planar molecular geometry, where the three oxygen atoms are arranged symmetrically around the central sulfur atom. After, marking electron pairs on atoms, we should mark charges of each atom. Sulfur trioxide readily acts as a Lewis acid to form addition compounds with many substances, but boron trifluoride is a much stronger Lewis acid than sulfur trioxide. In the above structure, there are charges on oxygen atoms and sulfur atom. In the Lewis definitions ofacids and bases, a Lewis acid is an electron pair 'acceptor,' which will acquire an electron pair. If you are a beginner to lewis structure drawing, follow these sections slowly and properly to understand it completely. The Sulfur atom does not have a lone pair, while all three Oxygen atoms have 2 lone pairs. There are three double bonds around sulfur atom with oxygen atoms in SO molecule. Most of the positive charge electron deficient areas, colored blue is concentrated on the S atom as it is expected from the Lewis structures of SO 3 and by chemical intuition. Negative electrostatic potential corresponds to a attraction of the proton by the concentrated electron density in the molecules mainly from lone pairs, pi-bonds,

The matchless phrase, is pleasant to me :)
Magnificent idea and it is duly