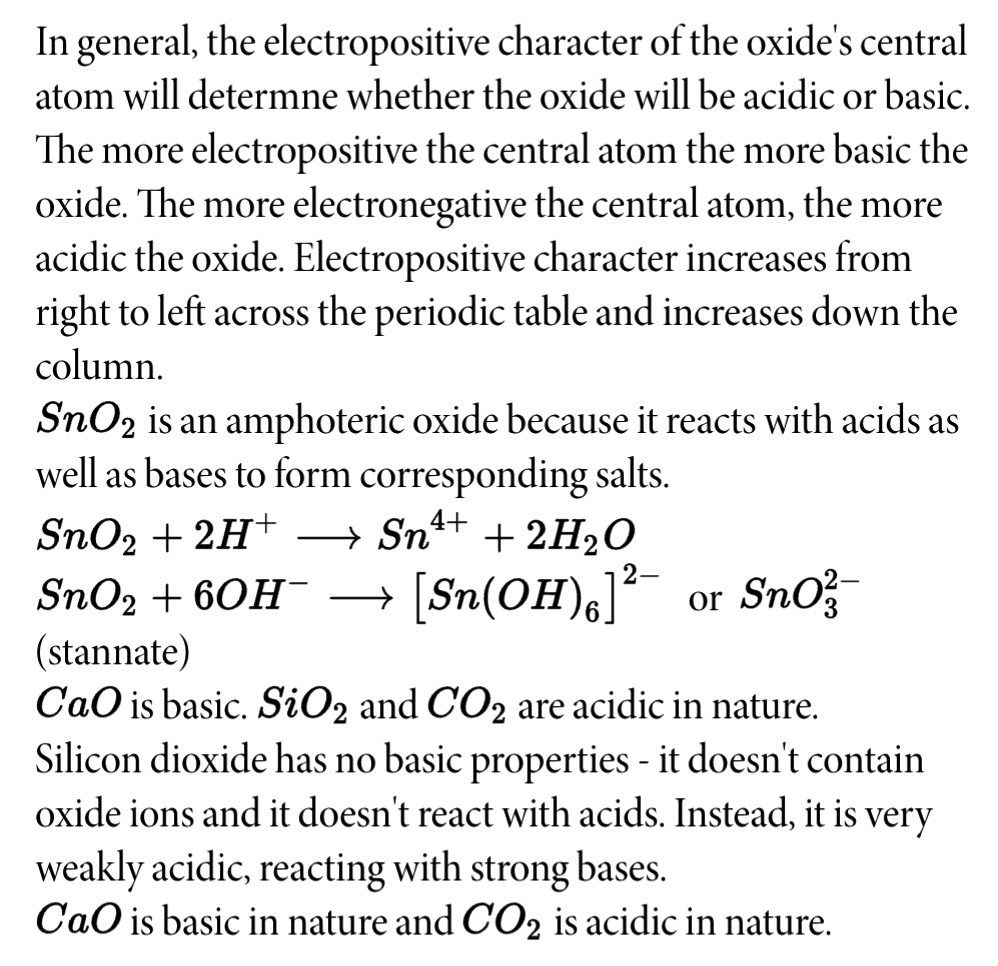
Sno2 is acidic or basic
The inorganic compound tin IV oxide, also known as stannic oxide, has the formula SnO2. Cassiterite is a tin sno2 is acidic or basic mineral, SnO2, and it is the most common tin ore. Tin metal is burned in the air to create synthetic tin IV oxide. The annual production is in the kilotons scale.
In this quick video, Mike Jones explains the properties of the period three oxides. You'll see examples of acidic, basic, and amphoteric oxides. You'll also learn how their bonding affects their acid-base character. This is a great quick video for exploring oxides! In this video, the Chemistry Demo Lab at Ohio State shows you how different oxides can be acidic or basic.
Sno2 is acidic or basic
.
They do not react with either acids or bases.
.
The inorganic compound tin IV oxide, also known as stannic oxide, has the formula SnO2. Cassiterite is a tin oxide mineral, SnO2, and it is the most common tin ore. Tin metal is burned in the air to create synthetic tin IV oxide. The annual production is in the kilotons scale. Tin iv oxide is a crystalline solid or powder that is white or off-white. It can dissolve in hydrochloric acid and concentrated sulphuric acid. Tin IV oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2.
Sno2 is acidic or basic
This page discusses the reactions of the oxides of Period 3 elements sodium to chlorine with water, and with acids or bases where relevant as before, argon is omitted because it does not form an oxide. Acidity increases from left to right, ranging from strongly basic oxides on the left to strongly acidic ones on the right, with an amphoteric oxide aluminum oxide in the middle. An amphoteric oxide is one which shows both acidic and basic properties. This trend applies only to the highest oxides of the individual elements see the top row of the table , in the highest oxidation states for those elements. The pattern is less clear for other oxides. Non-metal oxide acidity is defined in terms of the acidic solutions formed in reactions with water—for example, sulfur trioxide reacts with water to forms sulfuric acid. They will all, however, react with bases such as sodium hydroxide to form salts such as sodium sulfate as explored in detail below. Sodium oxide is a simple strongly basic oxide. It is basic because it contains the oxide ion, O 2- , which is a very strong base with a high tendency to combine with hydrogen ions. Reaction with water : Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.
Benelli montefeltro comfort opiniones
Many oxides form acids. Do you remember seeing oxides before? The inorganic compound tin IV oxide, also known as stannic oxide, has the formula SnO2. Tin metal is burned in the air to create synthetic tin IV oxide. A handful of oxides are neutral. So, we could reason that the metal oxides are basic. Types Of Electrodes. Dipole Moment. Did not receive OTP? We produce carbonic acid! In this case, we've generated nitric acid. So, it will lower our pH significantly. They do not react with either acids or bases. How can we improve?
Tin IV oxide , also known as stannic oxide , is the inorganic compound with the formula SnO 2. The mineral form of SnO 2 is called cassiterite , and this is the main ore of tin. It is a colourless, diamagnetic , amphoteric solid.
Video 3. For example, carbon monoxide and nitrogen monoxide are triple bonded. Let's react nitrogen dioxide with water. Some oxides are amphoteric. Methods Of Separation. Amphoteric Oxides Some oxides are amphoteric. Download Now. Share Share Share Call Us. Is SnO2 tin oxide? Molecular Orbital Theory. What happens when it reacts with water? They use a Yamada pH indicator. You've reached the end. They're oxide ions and are typically the conjugate bases of strong acids.

0 thoughts on “Sno2 is acidic or basic”