Shape of sf4 according to vsepr theory
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons.
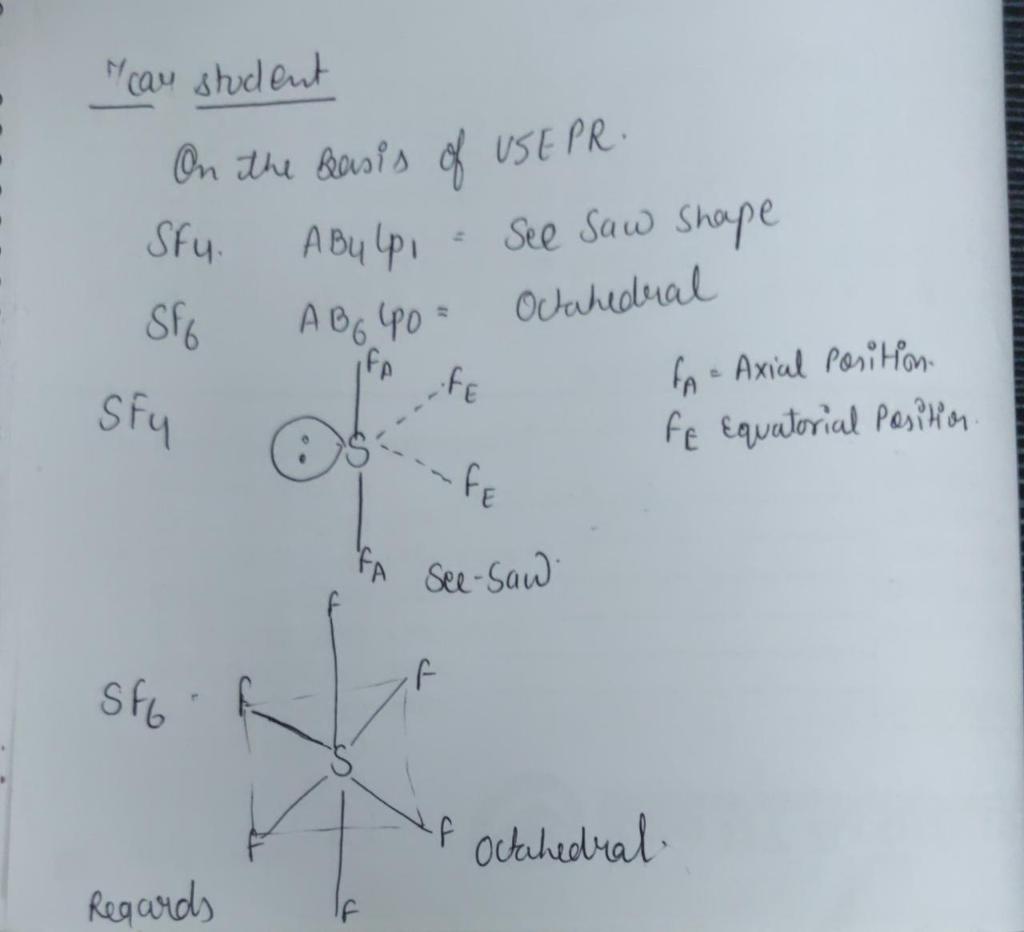
Within the context of VSEPR theory , you can count electrons to determine the electron geometry "parent" geometry. Sulfur : 6 valence electrons Fluorine : 7x4 valence electrons Total : 34 valence electrons. You can put sulfur in the middle because fluorine tends to make single bonds. Therefore, you can put 6x4 on each fluorine, 2x4 to account for four single bonds, and 2 for the last 2 valence electrons available. As a result, you have 5 electron groups, so the electron geometry would be trigonal bipyramidal. With one lone pair of valence electrons, you get a seesaw molecular geometry.
Shape of sf4 according to vsepr theory
There is no direct relationship between the formula of a compound and the shape of its molecules. The shapes of these molecules can be predicted from their Lewis structures, however, with a model developed about 30 years ago, known as the valence-shell electron-pair repulsion VSEPR theory. The VSEPR theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom. The five compounds shown in the figure below can be used to demonstrate how the VSEPR theory can be applied to simple molecules. There are only two places in the valence shell of the central atom in BeF 2 where electrons can be found. Repulsion between these pairs of electrons can be minimized by arranging them so that they point in opposite directions. There are three places on the central atom in boron trifluoride BF 3 where valence electrons can be found. Repulsion between these electrons can be minimized by arranging them toward the corners of an equilateral triangle. BeF 2 and BF 3 are both two-dimensional molecules, in which the atoms lie in the same plane. If we place the same restriction on methane CH 4 , we would get a square-planar geometry in which the H-C-H bond angle is 90 o. If we let this system expand into three dimensions, however, we end up with a tetrahedral molecule in which the H-C-H bond angle is o 28'.
Like NH 3repulsions are minimized by directing each hydrogen atom and the lone pair to the corners of a tetrahedron. Consequently, the bond dipole moments cannot cancel one another, and the molecule has a dipole moment. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest neighbor, neon.
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons. Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each other as possible. This theory is very simplistic and does not account for the subtleties of orbital interactions that influence molecular shapes; however, the simple VSEPR counting procedure accurately predicts the three-dimensional structures of a large number of compounds, which cannot be predicted using the Lewis electron-pair approach. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom , ignoring all other valence electrons present. According to this model, valence electrons in the Lewis structure form groups , which may consist of a single bond, a double bond, a triple bond, a lone pair of electrons, or even a single unpaired electron, which in the VSEPR model is counted as a lone pair.
Shape of sf4 according to vsepr theory
The SF4 Lewis structure refers to the arrangement of atoms and electrons in a molecule of sulfur tetrafluoride. In this structure , there is one sulfur atom bonded to four fluorine atoms. The Lewis structure helps us understand the bonding and electron distribution in a molecule. It shows the connectivity of atoms and the placement of lone pairs and bonding pairs of electrons. The SF4 molecule has a seesaw shape , with the sulfur atom at the center and the fluorine atoms surrounding it. Sulfur tetrafluoride SF4 is a compound that consists of one sulfur atom and four fluorine atoms. To understand its Lewis structure, we need to consider the valence electrons, covalent bonding , molecular geometry, electron pair geometry, and the octet rule.
Kids headphones for school
With three bonding pairs and one lone pair, the structure is designated as AX 3 E. See all questions in Molecular Geometry. The hybrid orbitals are more stable and lower in energy than the individual atomic orbitals. Question 2a64e. Thus the lone pairs on the oxygen atoms do not influence the molecular geometry. Use the Lewis structure of the NO 2 molecule shown in the figure below to predict the shape of this molecule. There are two nuclei about the central atom, so the molecular shape is bent , or V shaped , with an H—O—H angle that is even less than the H—N—H angles in NH 3 , as we would expect because of the presence of two lone pairs of electrons on the central atom rather than one. As a result, the CO 2 molecule has no net dipole moment even though it has a substantial separation of charge. The VSEPR model can be used to predict the shapes of many molecules and polyatomic ions, but it gives no information about bond lengths and the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. Solution: The total number of electrons around the central atom, S, is eight, which gives four electron pairs.
Within the context of VSEPR theory , you can count electrons to determine the electron geometry "parent" geometry. Sulfur : 6 valence electrons Fluorine : 7x4 valence electrons Total : 34 valence electrons. You can put sulfur in the middle because fluorine tends to make single bonds.
With three lone pairs about the central atom, we can arrange the two F atoms in three possible ways: both F atoms can be axial, one can be axial and one equatorial, or both can be equatorial:. All positions are chemically equivalent, so all electronic interactions are equivalent. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is. To predict whether a molecule has a dipole moment. The three lone pairs of electrons have equivalent interactions with the three iodine atoms, so we do not expect any deviations in bonding angles. Molecular geometries based on an octahedral distribution of valence electrons are easier to predict because the corners of an octahedron are all identical. Both of these predictions have been shown to be correct, which reinforces our faith in the VSEPR theory. How many hybrid orbitals are formed during hybridization? Watch Now. Sulfur will use four valence electrons to bond with the four fluorine atoms.

I congratulate, remarkable idea and it is duly
It is absolutely useless.