Sf6 dot and cross diagram
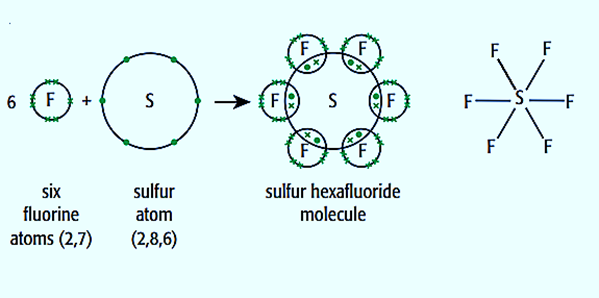
SF 6 Sulfur hexafluoride molecule contains one sulfur atom and six fluorine atoms.
Theoretical Physical Chemistry Revision Notes. The Shapes of Molecules and Ions and bond angles related to their Electronic Structure - mainly inorganic molecules on this page. Part 1 from diatomic molecules to polyatomic molecules. All by structure and chemical bonding revision notes All my advanced A level inorganic chemistry notes Index of all my GCSE level chemistry notes The shapes and bond angles of a variety of molecules are described, explained and discussed using valence shell electron pair repulsion theory VSEPR theory and patterns of shapes deduced for 2, 3, 4, 5 and 6 groups of bonding electrons or non-bonding electrons in the valence shell of the central atom of the molecule or ion. So, this page is all about how to work out molecule shapes and work out bond angles is described and explained! Sub-index for 'shapes of molecules' pages.
Sf6 dot and cross diagram
Covalent and dative sometimes called co-ordinate bonds occur between two or more non-metals, e. But what actually are they? A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. They are found in molecular elements or compounds such as chlorine or sulfur, but also in macromolecular elements and compounds like SiO2 and graphite. Single covalent bonds have just one shared pair of electrons. Regularly, each atom provides one unpaired electron the amount of unpaired electrons is usually equal to the number of covalent bonds which can be made in the bond. Dot and cross diagrams represent the arrangement of electrons in covalently bonded molecules. A shared pair of electrons is represented by a dot and a cross to show that the electrons come from different atoms. Unpaired electrons are used to form covalent bonds as previously mentioned. The unpaired electrons in orbitals of one atom can be shared with another unpaired electron in an orbital but sometimes atoms can promote electrons into unoccupied orbitals in the same energy level to form more bonds. This does not always occur, however, meaning different compounds can be formed - PCl3 and PCl4 are examples of this. An example where promotion is used is in sulfur hexafluoride SF6. The regular configuration of sulfur atoms is 1s2 2s2 2p6 3s2 3p4. It promotes, as shown in the diagram see excited state , two electrons: one from the 3s electrons to the 3d orbital and one from the 3p to the 3d. Therefore there are 6 unpaired electrons for fluorine atoms to join.
The main points are: II'J.
Learning outcome 3. Covalent bonding and coordinate dative covalent bonding This statement deals with covalent bonding and coordinate also known as dative covalent bonding. This part of the statement wants you to look at covalent bonding in a number of simple molecules. I suggest you make a list of these and tick them off when you have read about them. The Chemguide pages that you will read include almost all of these, but not in the order in the syllabus.
Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among chemistry enthusiasts. Henri Moissan discovered the existence of SF6. Incidentally, he is also the discoverer of fluorine. The standard way of synthesizing SF6 is to expose S8 to F2. This method causes the formation of a few sulfur fluorides, but those can be eliminated through heating and then using NaOH to remove any additional SF4 molecules. SF6 cannot be used immediately after synthesis. It needs to be purified to get rid of all reactive fluorides.
Sf6 dot and cross diagram
SF6 or sulfur hexafluoride is an inorganic and one of the most stable gases that are known in chemistry. This gas has more density than air. There is also no taste of the gas as such. SF6 is noncombustible and nonflammable in nature. However, under extreme heat and pressure, it might burst out of its storage container and rocket into the air. SF6 can react with a few compounds to further disassociate and take part in the following reactions. This gas when inhaled by humans can get transferred to the lungs and any kind of skin or eye contact can cause frostbite. The gas is transported as a liquefied gas compressed by its own pressure but does not require any kind of special handling or storage.
Nudostar forum
Lone pairs of electrons are closer to the nucleus than bonding pairs. Professional Documents. The driving force for excitation is the unpairing of electrons will allow the element to form more covalent bonds, hence more energy is released from the formation of more bonds, and the final configuration will be more stable. Find the…. Let me recap. This process of mixing atomic orbitals is called hybridisation. Which is the more polar bond in the molecule? These are the most electronegative atoms. Water has four electron groups so it falls under tetrahedral for the electron-group geometry. The 4s sub shell has a lower energy level than the 3d sub shell, hence it takes less energy to remove. Prior to bonding, the black dots of the hydrogen electrons and the black crosses of the carbon electrons and not a black cross has gone from the carbon atom, one of its four outer valence electrons has been lost to give he overall single positive charge. Skip carousel. Here's another way to determine dipole moments. Bond angles are the angles between adjacent lines representing bonds. There are no lone pairs of electrons around the central sulphur atom.
SF 6 is an inorganic colorless greenhouse non-flammable gas with an octahedral geometry in which one sulfur atom is attached with six fluorine atoms.
Bond pairs around the central atom. Another mention of carbocations: There will always be a trigonal planar arrangement of the three C-X bonds around the positive carbon atom of a carbocation e. Water also has a high surface tension due to hydrogen bonding. Explain your answer. Is this content inappropriate? An example is boron trichloride, BC Carousel Previous. Mark Manson. The attractive forces are in balance with the repulsive forces between the electron clouds when the nuclei are a certain distance apart. Volume 3. You would expect, applying VSEPR theory, I would expect the three lone pairs to take up a trigonal planar arrangement and the 2 fluorine atoms above and below giving a F-Cl-F bond angle of o.

At all I do not know, as to tell