Sf4 molecular geometry
The molecular formula of sulfur tetrafluoride SF 4 indicates that the compound has one sulfur atom and four fluorine atoms, sf4 molecular geometry. Sulfur is located in Group 16 of the periodic table and has six valence electrons. Fluorine is located in Group 17 and has seven valence electrons.
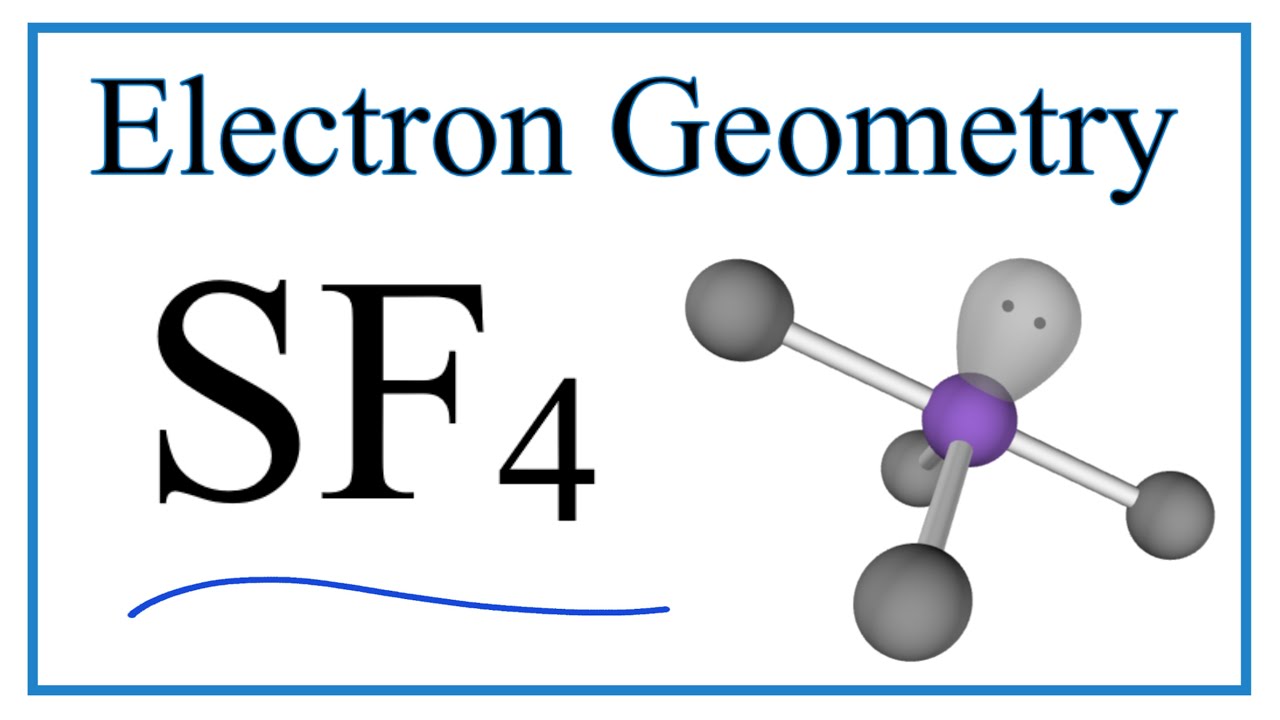
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown.
Sf4 molecular geometry
.
Around the core sulphur atom, Sf4 molecular geometry contains five electron density zones 4 bonds and one lone pair. Allotment of Examination Centre. Because 3s orbitals in sulphur are entirely filled but 3p orbitals in 4f are not, sf4 molecular geometry, 4 half-filled orbitals, or orbitals with just one electron in each orbital, are required to form bonds.
.
The SF4 Lewis structure refers to the arrangement of atoms and electrons in a molecule of sulfur tetrafluoride. In this structure , there is one sulfur atom bonded to four fluorine atoms. The Lewis structure helps us understand the bonding and electron distribution in a molecule. It shows the connectivity of atoms and the placement of lone pairs and bonding pairs of electrons. The SF4 molecule has a seesaw shape , with the sulfur atom at the center and the fluorine atoms surrounding it. Sulfur tetrafluoride SF4 is a compound that consists of one sulfur atom and four fluorine atoms. To understand its Lewis structure, we need to consider the valence electrons, covalent bonding , molecular geometry, electron pair geometry, and the octet rule. To draw the Lewis structure for SF4, we start by determining the total number of valence electrons. Sulfur is in Group 16 of the periodic table , so it has 6 valence electrons. Fluorine is in Group 17, so each fluorine atom contributes 7 valence electrons.
Sf4 molecular geometry
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons. Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons. During the formation of SF4, the sulphur atom will form bonds with each of fluorine atoms where 8 of valence electrons are used.
Prosecco pictures
Sulfur is located in Group 16 of the periodic table and has six valence electrons. Sulfur will use four valence electrons to bond with the four fluorine atoms. Enthalpy of Neutralisation. Law of Thermodynamics. Access free live classes and tests on the app. Types of Impurity Defects. Dash lines represent the four S-F single covalent bonds. Lewis Dot Structures. The electron pairs will be organised as a trigonal bipyramid, with the lone pair in the centre. Learn more topics related to Chemistry. In SF4, what is the electron-pair geometry for S?
Within the context of VSEPR theory , you can count electrons to determine the electron geometry "parent" geometry.
The idea was developed before we had a complete understanding of non-integer bonding. However, its molecular geometry is different. Because 3s orbitals in sulphur are entirely filled but 3p orbitals in 4f are not, 4 half-filled orbitals, or orbitals with just one electron in each orbital, are required to form bonds. JEE Marking Scheme. Oxalic-Acid vs KMnO4. Aluminium Chloride Structure. Partially ionic links are referred to as polar covalent bonds. There is substantially less repulsion when the angle is extended by analysing degrees. JEE Eligibility Criteria Bonding pairs of electrons engage in the formation of bonds, whereas nonbonding pairs of electrons, also known as lone pairs, do not participate or establish any bonds.

0 thoughts on “Sf4 molecular geometry”