Sf4 bond angle
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order sf4 bond angle determine the hybridization of sulphur tetrafluoride, sf4 bond angle, you have to first understand its Lewis structure and the number of valence electrons that are present.
The process of mixing of atomic orbitals belonging to the same atom of slightly different energies so that a redistribution of energy takes place between them resulting in the formation of new sets of orbitals of equivalent energies and shape is called hybridization. The new orbitals in this form are known as hybrid orbitals. Like pure orbitals the hybrid orbitals are used in Bond formation. Hybridization is a hypothetical concept and has been introduced in order to explain the characteristic geometrical shapes of polyatomic molecules. The central atom is S. So, to explain in simple terms, its bonding regions are four having one lone pair. There are 34 valence electrons and 5 electron pairs.
Sf4 bond angle
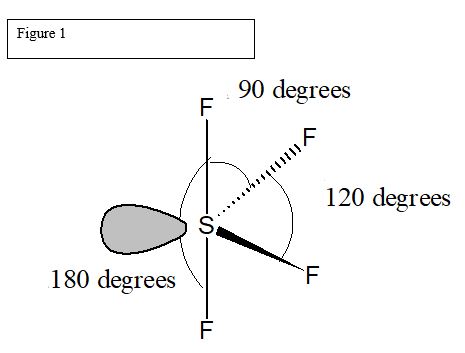
Within the context of VSEPR theory , you can count electrons to determine the electron geometry "parent" geometry. Sulfur : 6 valence electrons Fluorine : 7x4 valence electrons Total : 34 valence electrons. You can put sulfur in the middle because fluorine tends to make single bonds. Therefore, you can put 6x4 on each fluorine, 2x4 to account for four single bonds, and 2 for the last 2 valence electrons available. As a result, you have 5 electron groups, so the electron geometry would be trigonal bipyramidal. With one lone pair of valence electrons, you get a seesaw molecular geometry. Note though that the structure is distorted a bit due to the repulsive forces of the lone pair of electrons you see not bonded. So, that bends the axial fluorines together a bit. What is the shape of SF4 including bond angles? Truong-Son N.
Open in App. Reserved Seats. Ans : Seesaw is the shape.
Hybridization of SF4 is sp 3 d. In this hybridization, a total of five hybrid orbitals are formed. Sulfur tetrafluoride, or SF4 is a chemical compound made up of four fluorine atoms and one sulfur atom. It is a colorless compound that releases poisonous HF gas when reacts with water or moisture. In this article, we will learn about the hybridization of SF4 and its geometry with a brief introduction to the SF4 molecule and the hybridization process. Sulfur Tetrafluoride is a gaseous compound that consists of one sulfur atom and is bonded to four fluorine atoms. It is a colorless compound and when reacts with water or moisture releases Hydrogen Fluoride gas.
Thionyl tetrafluoride , also known as sulfur tetrafluoride oxide , is an inorganic compound with the formula S O F 4. It is a colorless gas. The shape of the molecule is a distorted trigonal bipyramid, with the oxygen found on the equator. The atoms on the equator have shorter bond lengths than the fluorine atoms on the axis. The sulfur oxygen bond is 1. The angle between the equatorial fluorine atoms is The angle between axial fluorine and oxygen is The angle between oxygen and equatorial fluorine is Thionyl fluoride reacting with fluorine gas can produce thionyl tetrafluoride.
Sf4 bond angle
The SF4 Lewis structure refers to the arrangement of atoms and electrons in a molecule of sulfur tetrafluoride. In this structure , there is one sulfur atom bonded to four fluorine atoms. The Lewis structure helps us understand the bonding and electron distribution in a molecule. It shows the connectivity of atoms and the placement of lone pairs and bonding pairs of electrons. The SF4 molecule has a seesaw shape , with the sulfur atom at the center and the fluorine atoms surrounding it. Sulfur tetrafluoride SF4 is a compound that consists of one sulfur atom and four fluorine atoms.
T mobile stores lancaster pa
Learn more. Ans : Not all ligands peripheral groups in trigonal bipyramidal molecules or complexes are equidi The 3-dimensional arrangement of atoms or fragments which create a molecule by getting together is called Molecular Geometry. Sulphur is a periodic table group VIA element with six electrons in its final shell valence shell. Create Improvement. Lewis Dot Structures. The middle S atom containing the 5 valence atomic orbitals is basically hybridized to form five sp 3 d hybrid orbitals. In this hybridization, a total of five hybrid orbitals are formed. We also learn the importance of XeF6 molecular geometry and bond angles importance and much more about the topic in detail. The shape of water molecule which should be tetrahedral has a bent or distorted tetrahedral shape with a bond angle Law of Thermodynamics. The hybrid orbitals are more stable and lower in energy than the individual atomic orbitals.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons.
Read full. Sulphur is a periodic table group VIA element with six electrons in its final shell valence shell. Molecular Weight. There is substantially less repulsion when the angle is extended by analysing degrees. Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. See all questions in Molecular Geometry. In SF4, how many lone pairs of electrons are there on the S atom? Statistics Cheat Sheet. Get subscription. Types of Impurity Defects.

I think, that you are not right.
I consider, that you are not right. I can prove it. Write to me in PM, we will communicate.
I think, that you are not right. I can prove it. Write to me in PM, we will discuss.