Sf2 hybridization
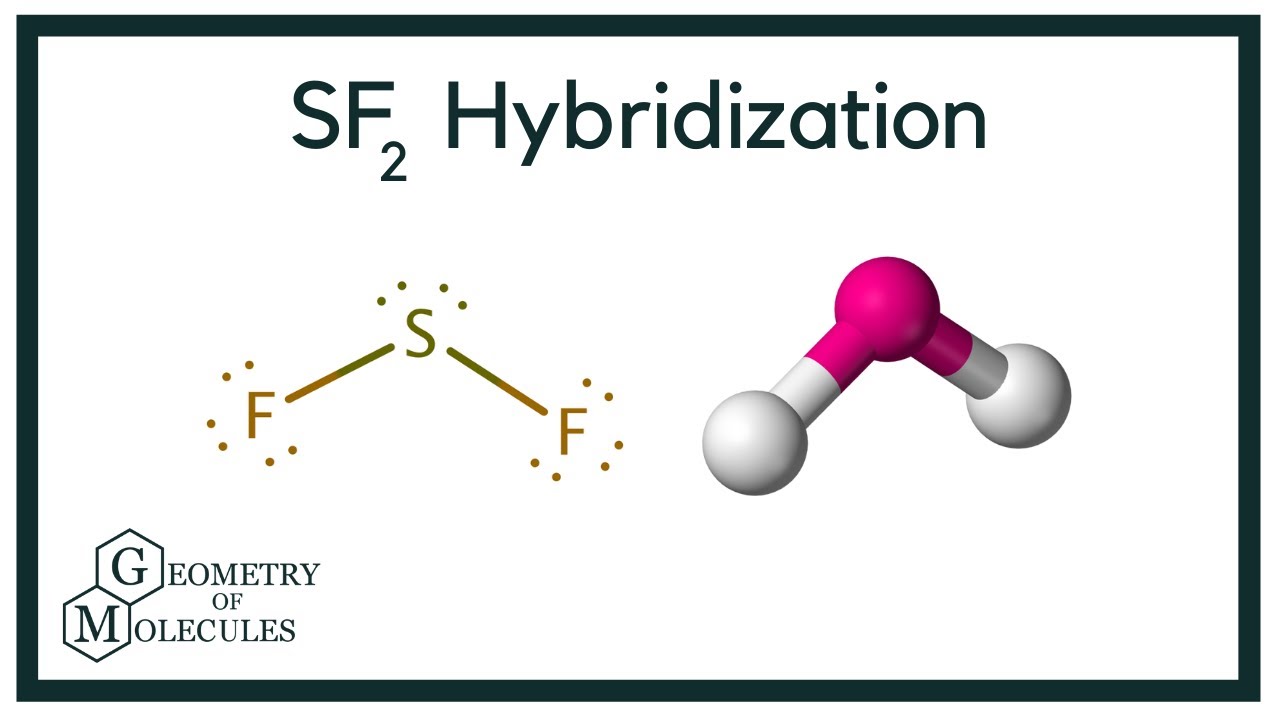
Sulfur Difluoride is an inorganic sf2 hybridization made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF 2its molecular geometry and shape.
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride. Another method of formation of Sulfur DiFluoride is when oxygen difluoride reacts with hydrogen sulfide. Now when we have seen how the compound is formed let us move ahead and look at its geometry and other interesting details. Lewis Structure is nothing but an arrangement of valence electrons between different atoms.
Sf2 hybridization
.
And, the valence electrons of Fluorine are 7 in number.
.
Sulfur difluoride SF2 has the composition of one sulfur and two fluorine atoms. What is the molecular geometry of sulfur difluoride?. Drawing and predicting the SF2 molecular geometry is very easy by following the given method. Here in this post, we described step by step to construct SF2 molecular geometry. Sulfur and fluorine come from the 16th and 17th family groups in the periodic table. Sulfur and fluorine have six and seven valence electrons respectively. A three-step approach for drawing the SF2 molecular can be used. The first step is to sketch the molecular geometry of the SF2 molecule, to calculate the lone pairs of the electron in the central sulfur atom; the second step is to calculate the SF2 hybridization, and the third step is to give perfect notation for the SF2 molecular geometry. The SF2 molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the SF2 molecule in a specific geometric manner.
Sf2 hybridization
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to o , o , or o. According to Valence Shell Electron Pair Repulsion VSEPR theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Carbon is a perfect example showing the value of hybrid orbitals. Carbon's ground state configuration is:. According to Valence Bond Theory , carbon should form two covalent bonds, resulting in a CH 2 , because it has two unpaired electrons in its electronic configuration. Therefore, this does not explain how CH 4 can exist.
Socialblade mr beast
So it will be in the central position with both these Fluorine atoms on the terminal ends. We access the lone pairs on the atom, the shape, and other things while finding the polarity of a compound. So Sulfur Difluoride has a bent molecular geometry. Skip to content Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. According to VSEPR theory, each atom in a compound is arranged in a way that the compound becomes stable in nature. These configurations are decided on the basis of the number of electrons these elements have. Bond Angle of SF2. Valence electrons of Sulfur are 6 in number. So we will first find out the total valence electrons for Sulphur Difluoride. There are in total 2 atoms of Fluorine in this compound. To determine the polarity of any molecule, we check for the following factors:. Another way of finding the hybridization of SF2 is by calculating the steric number of SF2, which we can find by the following equation. First, the electrons are filled in 1s, then in 2s, and so on. And if not writing you will find me reading a book in some cosy cafe!
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF 2 , its molecular geometry and shape.
In fact, both Fluorine atoms are also sp3 hybridized. To read, write and know something new every day is the only way I see my day! Save my name, email, and website in this browser for the next time I comment. Due to the presence of the lone pairs, there is symmetry in the molecule. The direction of the dipole moment will be from the Sulphur atom towards the Fluorine atom, as here Fluorine will try to pull the shared electrons to itself. And so on…. To find out the Hybridization of this molecule, we will consider the two numbers of atoms and the total number of lone electron pairs bonded to the molecule. In this blog post, we will look at the Lewis dot structure of SF 2 , its molecular geometry and shape. November 23, First, we will have to calculate the total number of valence electrons in this compound. To determine the polarity of any molecule, we check for the following factors:. As this molecule is not linear, the dipole moments on both sides are not canceled out, resulting in the non-zero net dipole moment of the molecule.

I am sorry, that has interfered... At me a similar situation. I invite to discussion.
It is a pity, that I can not participate in discussion now. I do not own the necessary information. But with pleasure I will watch this theme.
Completely I share your opinion. In it something is also I think, what is it excellent idea.