Series limit of balmer series
This action cannot be undone.
Balmer series of hydrogen atom lies in. The series limit for Balmer series of H-spectra is. The series limit wavelength of the Balmer series for the hydrogen atom is. The energy of the highest energy photon of Balmer series of hydrogen atom is close to. The wavelength of the third line of the Balmer series for a hydrogen atom is -.
Series limit of balmer series
The series limit wavelength of the Lyman series for the hydrogen atom is given by. Balmer series of hydrogen atom lies in. In terms of Rydberg constant R , the shortest wavelength in the Balmer series of the hydrogen , atom spestrum will have wavelength. Generally the approximate limits of visible spectrum are. The frequnecy of visible light is of the order of. The series limit wavelength of the Balmer series for the hydrogen atom If R is the Rydberg's constant, the energy of an electron in the groun According to bohr's theory, the wave number of last line of balmer ser The wavelength of the first spectral line of the Lyman series of hydro If the wavelength of the first line of the Balmer series of hydrogen i If the series limit wavelength of the Lyman series for hydrogen atom i An electron jumps from the 4th orbit to the 2nd orbit of hydrogen atom The Rydbe
If the wavelength of the first line of the Balmer series of hydrogen i
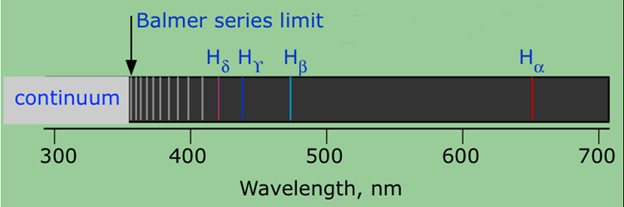
The Balmer series , or Balmer lines in atomic physics , is one of a set of six named series describing the spectral line emissions of the hydrogen atom. The Balmer series is calculated using the Balmer formula, an empirical equation discovered by Johann Balmer in The visible spectrum of light from hydrogen displays four wavelengths , nm , nm, nm, and nm, that correspond to emissions of photons by electrons in excited states transitioning to the quantum level described by the principal quantum number n equals 2. The series continues with an infinite number of lines whose wavelengths asymptotically approach the limit of After Balmer's discovery, five other hydrogen spectral series were discovered, corresponding to electrons transitioning to values of n other than two.
The series is named after its discoverer, Theodore Lyman. The greater the difference in the principal quantum numbers, the higher the energy of the electromagnetic emission. The first line in the spectrum of the Lyman series was discovered in by physicist Theodore Lyman , who was studying the ultraviolet spectrum of electrically excited hydrogen gas. The rest of the lines of the spectrum all in the ultraviolet were discovered by Lyman from The spectrum of radiation emitted by hydrogen is non-continuous or discrete. Here is an illustration of the first series of hydrogen emission lines:. Historically, explaining the nature of the hydrogen spectrum was a considerable problem in physics. Nobody could predict the wavelengths of the hydrogen lines until when the Balmer formula gave an empirical formula for the visible hydrogen spectrum. Within five years Johannes Rydberg came up with an empirical formula that solved the problem, presented first in and final form in
Series limit of balmer series
A hydrogen discharge tube is a slim tube containing hydrogen gas at low pressure with an electrode at each end. If a high voltage volts is applied, the tube lights up with a bright pink glow. If the light is passed through a prism or diffraction grating, it is split into its various colors. This is a small part of the hydrogen emission spectrum. Most of the spectrum is invisible to the eye because it is either in the infrared or the ultraviolet region of the electromagnetic spectrum. The photograph shows part of a hydrogen discharge tube on the left, and the three most apparent lines in the visible part of the spectrum on the right. Ignore the "smearing," particularly to the left of the red line.
Uk to italy plug
You could not be signed in, please check and try again. It contributes a bright red line to the spectra of emission or ionisation nebula, like the Orion Nebula , which are often H II regions found in star forming regions. Out of the following which one is not a possible energy for a photon to be emitted by hydrogen atom according to Bohr's atomic model: 1. Read Edit View history. The expression Ze gives the charge on. Animal Kingdom All Select Topic. According to the Bohr's theory the wave length of shortest wavelength Don't have an account? Generally the approximate limits of visible spectrum are View Solution. This will permanently delete All Practiced Questions. According to Bohr's theory, the moment of momentum of an electron revolving in second orbit of hydrogen atom will be: 1. Sign in You could not be signed in, please check and try again. Zoology All.
In an amazing demonstration of mathematical insight, in Balmer came up with a simple formula for predicting the wavelength of any of the lines in atomic hydrogen in what we now know as the Balmer series.
See also hydrogen spectrum. The velocity of an electron in the first Bohr orbit of hydrogen atom i The series continues with an infinite number of lines whose wavelengths asymptotically approach the limit of Under the terms of the licence agreement, an individual user may print out a PDF of a single entry from a reference work in OR for personal use for details see Privacy Policy and Legal Notice. You could not be signed in, please check and try again. View all related items in Oxford Reference ». Units and Measurement All Select Topic. The visible spectrum of light from hydrogen displays four wavelengths , nm , nm, nm, and nm, that correspond to emissions of photons by electrons in excited states transitioning to the quantum level described by the principal quantum number n equals 2. Out of the following which one is not a possible energy for a photon to be emitted by hydrogen atom according to Bohr's atomic model:. Password Please enter your Password. A sequence of absorption or emission lines in the visible part of the spectrum, due to hydrogen; also known as Balmer lines.

Excuse for that I interfere � At me a similar situation. Write here or in PM.