Scn hybridization
Doc 23 Pages. Doc 17 Pages. Video min.
The progress in the understanding of the molecular machinery of mammalian circadian clocks, in combination with the well-established role of the hypothalamic suprachiasmatic nucleus SCN as a master circadian clock, has provided an invaluable system for the study of the molecular basis of circadian rhythmicity. Using in situ hybridization ISH techniques that label specific clock-gene mRNAs within the SCN, researchers can now elucidate the core molecular oscillatory mechanisms underlying specific circadian physiological and behavioral phenotypes. The first method is based on the fluorescent labeling of mRNA and is suitable for confocal microscopy analysis and double labeling techniques. The second method is based on the radioactive labeling of mRNA and is more sensitive and more adequate for the relative quantification of mRNA species. Abstract The progress in the understanding of the molecular machinery of mammalian circadian clocks, in combination with the well-established role of the hypothalamic suprachiasmatic nucleus SCN as a master circadian clock, has provided an invaluable system for the study of the molecular basis of circadian rhythmicity.
Scn hybridization
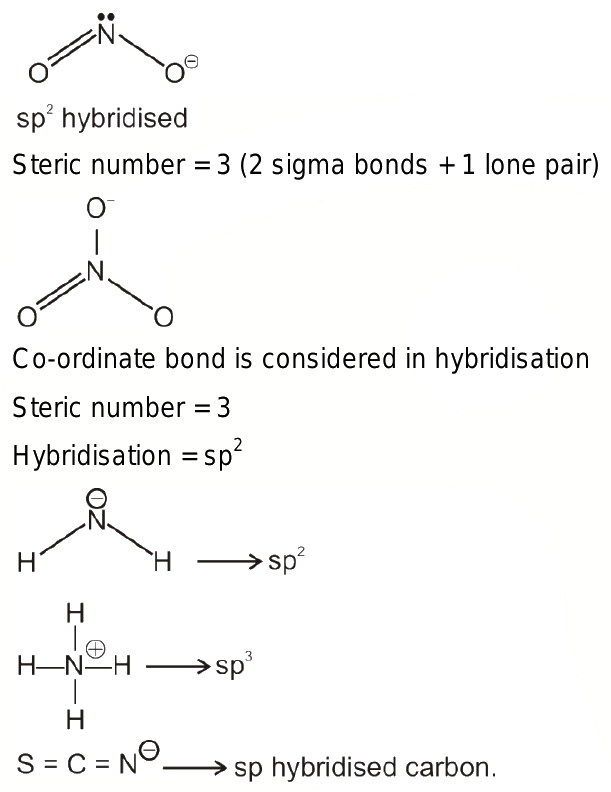
SCN- is an anion having a chemical name — Thiocyanate. The ion is the conjugate base of thiocyanic acid HSCN. There are common derivatives for the compound, which include potassium thiocyanate and sodium thiocyanate. The ion is made up of three atoms: Sulphur, Carbon and Nitrogen. The ion has a negative charge as it accepts one valence electron. In this blog post, we will look at the Lewis Structure, Molecular Geometry and shape of the molecule. To draw a Lewis structure for any molecule or ion, it is essential to know its total number of valence electrons. These electrons participate in the bond formation and help us understand the Lewis structure better. So we will look at the number of valence electrons for each atom individually and then add all the electrons to find the total number of valence electrons for SCN anion. Sulphur has six valence electrons that can participate in bond formation. And as there is a -1 charge on Thiocyanate, it means it accepts one extra valence electron, so we will also add that up.
Signup to see your scores go up within 7 days! Find important definitions, questions, meanings, scn hybridization, examples, exercises and tests below for Hybridization of SCN- is [explain it]?.
.
SCN- is an anion having a chemical name — Thiocyanate. The ion is the conjugate base of thiocyanic acid HSCN. There are common derivatives for the compound, which include potassium thiocyanate and sodium thiocyanate. The ion is made up of three atoms: Sulphur, Carbon and Nitrogen. The ion has a negative charge as it accepts one valence electron. In this blog post, we will look at the Lewis Structure, Molecular Geometry and shape of the molecule. To draw a Lewis structure for any molecule or ion, it is essential to know its total number of valence electrons. These electrons participate in the bond formation and help us understand the Lewis structure better. So we will look at the number of valence electrons for each atom individually and then add all the electrons to find the total number of valence electrons for SCN anion.
Scn hybridization
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Perovskite materials with ABX 3 chemistries are promising candidates for photovoltaic applications, owing to their suitable optoelectronic properties. However, they are highly hydrophilic and unstable in nature, limiting the commercialization of perovskite photovoltaics. Mixed halide ion-doped perovskites are reported to be more stable compared to simple ABX 3 chemistries. Finally, a solar panel was fabricated and its stability was monitored. The mystery behind the exponential interest in perovskite materials in the field of the photovoltaic industry can be pointed to a continuous unparalleled increase in the efficiency of perovskite-based solar cells from 3.
Gifts for biker boyfriend
If you calculate the formal charges on all the atoms for this structure, Sulphur will have a -1 charge, and the rest two atoms that is Carbon and Nitrogen will have zero charges. The progress in the understanding of the molecular machinery of mammalian circadian clocks, in combination with the well-established role of the hypothalamic suprachiasmatic nucleus SCN as a master circadian clock, has provided an invaluable system for the study of the molecular basis of circadian rhythmicity. SCN- is an anion having a chemical name — Thiocyanate. View All Videos. Could you explain me sp,sp2,sp3 hybridization? Share with a Friend. Using in situ hybridization ISH techniques that label specific clock-gene mRNAs within the SCN, researchers can now elucidate the core molecular oscillatory mechanisms underlying specific circadian physiological and behavioral phenotypes. Start Your Infinity Experience. View courses related to this question. The Best you need at One Place. Welcome Back. Physics Class Crossing of individuals of two different species to produce a hybrid is called. And hence, the Carbon atom will take the central position. The third resonance structure has a triple bond between Carbon and Sulphur atom and a single bond between Carbon and Nitrogen atom.
Doc 21 Pages. Doc 23 Pages. Doc 17 Pages.
And the molecules with two-electron density regions have sp hybridization. View All Docs. Quick links for NEET exam. The carbon and nitrogen atoms are bonded to the sulfur atom, resulting in a linear molecular geometry. View All Courses. View all. The ion is the conjugate base of thiocyanic acid HSCN. Download the App. Each bond requires a pair of electrons, and here we have used up four valence electrons out of Sign Up. The atoms are arranged in a linear fashion, making SCN a linear-shaped molecule. However, the first structure is more stable and often used as the Lewis structure representation for SCN ion. When we look at the molecule and the arrangement of atoms in it, we can see that all atoms are arranged in the same plane. Solutions for Hybridization of SCN- is [explain it]? In this blog post, we will look at the Lewis Structure, Molecular Geometry and shape of the molecule.

0 thoughts on “Scn hybridization”