Scl2 lewis structure
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m.
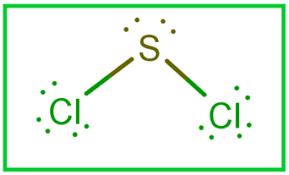
Sulfur dichloride SCl 2 contains one sulfur atom and two chlorine atoms. Lewis structure of SCl 2 contains only two S-Cl bonds. There are two lone pairs on sulfur atom and three lone pairs on each chlorine atom in SCl 2 lewis structure. Both chlorine atoms have made single bonds with sulfur atom. Also, there are three lone pairs exist on both chlorine atoms and two lone pairs on sulfur atom. When we draw lewis structures, there are several guidelines to follow.
Scl2 lewis structure
.
Equilibrium Constant K.
.
Sulfur dichloride is a red viscous liquid at room temperature. It has a pungent chlorine-like odor. It reacts with water to form chlorine-containing acids. SCl2 is a very corrosive and toxic substance. It is a covalent compound because the electronegativity difference between S and Cl is not significant. They both are non-metals. In this article, we will understand the concepts of Lewis dot structure, hybridization, and polarity.
Scl2 lewis structure
SCl 2 sulfur dichloride has one sulfur atom and two chlorine atoms. In the SCl 2 Lewis structure, there are two single bonds around the sulfur atom, with two chlorine atoms attached to it. Each chlorine atom has three lone pairs, and the sulfur atom has two lone pairs. In the periodic table , sulfur lies in group 16, and chlorine lies in group Hence, sulfur has six valence electrons and chlorine has seven valence electrons. Learn how to find: Sulfur valence electrons and Chlorine valence electrons. We have a total of 20 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
Electron withdrawing groups list
Born Haber Cycle. The Electron Configurations: Exceptions. Arrhenius Equation. Naming Benzene. Titrations: Weak Acid-Strong Base. Periodic Table: Group Names. Main Group Elements: Bonding Types. Diprotic Acids and Bases. De Broglie Wavelength. Phase Diagrams. Naming Ionic Hydrates. Maxwell-Boltzmann Distribution. Millikan Oil Drop Experiment. Electron Geometry. Chemical Bonds.
Transcript: This is Dr. Let's do the SCl2 Lewis structure. On the periodic table, Sulfur—group 6 or 16—has 6 valence electrons.
Periodic Trend: Electronegativity. Naming Cyclic Alkanes. Bronsted-Lowry Acids and Bases. Hybridization Example 1. Amphoteric Species. The Ideal Gas Law: Density. Lewis Dot Structures: Neutral Compounds. Root Mean Square Speed. Intro to Acid-Base Titration Curves. Clausius-Clapeyron Equation.

Absolutely with you it agree. In it something is also to me your idea is pleasant. I suggest to take out for the general discussion.