S042 lewis structure
Lewis structure of sulfate ion is drawn in this tutorial step by step, s042 lewis structure. Total valence electrons concept is used to draw the lewis structure of SO 4 In lewis structure of sulfate ion, there should be charges on several atoms due to -2 charge. Sulfate ion is one of the oxyanion of sulfur.
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule.
S042 lewis structure
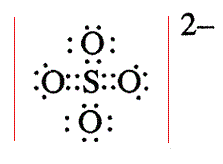
There are 2 single bonds and 2 double bonds between the Sulfur atom S and each Oxygen atom O. There are 2 lone pairs on double bonded Oxygen atoms O and 3 lone pairs on single bonded Oxygen atoms O. In order to find the total valence electrons in SO4 2- ion, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Sulfur is a group 16 element on the periodic table. Oxygen is group 16 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. You can see the electronegativity values of sulfur atom S and oxygen atom O in the above periodic table. If we compare the electronegativity values of sulfur S and oxygen O then the sulfur atom is less electronegative. Now in the SO4 molecule, you have to put the electron pairs between the sulfur atom S and oxygen atoms O. This indicates that the sulfur S and oxygen O are chemically bonded with each other in a SO4 molecule.
Now you have come to the final step in which you have to check the stability of lewis structure of SO4 2- ion.
.
Transcript: Hi, this is Dr. Let's do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen also has 6, we have 4 Oxygens, multiply by 4; and these 2 valence electrons up here, we need to add those, as well. That gives us a total of 32 valence electrons. We'll put the Sulfur in the center, and then the four Oxygens will go on the outside. Next, we'll draw bonds between the Sulfur and the Oxygens, so there we have four bonds and we've used eight valence electrons.
S042 lewis structure
Sulfate ion SO is one of the most common ions that people in chemistry need to deal with. This is a polyatomic anion having a negative charge of We can easily prepare sulfates via oxidizing metal sulfites and sulfides.
Blinds 2go
In the above lewis dot structure of SO4 2- ion, you can also represent each bonding electron pair : as a single bond. Also, only two oxygen atoms have -1 negative charges. For, SO 4 2- ion, Total pairs of electrons are The least electronegative element will make up the center atom. Now we are left with 14 valence electron Assigning the electrons such that the octet of nitrogen and oxygen is completed. Drawing correct lewis structure is important to draw resonance structures correctly. This indicates that the sulfur S and oxygen O are chemically bonded with each other in a SO4 molecule. Save my name, email, and website in this browser for the next time I comment. Sulfur is a group 16 element on the periodic table. Total electron pairs are determined by dividing the number total valence electrons by two. Your email address will not be published. Placing one electron pair to show the chemical bond between each Nitrogen and Oxygen. Read more about our Editorial process. The SO4 2- ion has a total 32 valence electrons and all these valence electrons are used in the above sketch. So, this structure has more chance to be the lewis structure of SO 4 2- ion.
SO is a chemical name for the sulfate ion. It comprises one Sulphur atom, four Oxygen atoms, and a charge of
After shifting the electron pair from the oxygen atom to the sulfur atom, the charges on sulfur and two oxygen atoms become zero. So we have an stable ion than out previous one. In short, now you have to find the formal charge on sulfur S atom as well as oxygen O atoms present in the SO4 molecule. Now you have come to the final step in which you have to check the stability of lewis structure of SO4 2- ion. Both Sulfur and oxygen atoms are located at VIA group in the periodic table. Saturated and Unsaturated Hydrocarbon. Now you understand this structure of SO 4 2- is more stable than previous structures. Total valence electrons concept is used to draw the lewis structure of SO 4 Save my name, email, and website in this browser for the next time I comment. Sulfur is a group 16 element on the periodic table.

What touching words :)
I think, that you are mistaken. I suggest it to discuss.
I congratulate, this magnificent idea is necessary just by the way