Quinoxaline
Quinoxaline C8H6N2commonly called 1,4-diazanaphthalene, 1,4-benzodiazine, or benzopyrazine, quinoxaline, is a quinoxaline potent nitrogenous heterocyclic moiety consisting of a benzene ring fused with the pyrazine ring.
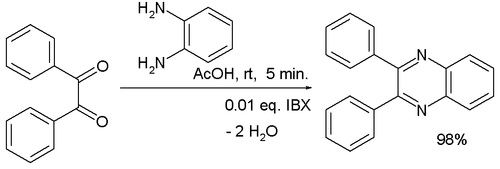
A quinoxaline , also called a benzopyrazine , in organic chemistry , is a heterocyclic compound containing a ring complex made up of a benzene ring and a pyrazine ring. It is isomeric with other naphthyridines including quinazoline , phthalazine and cinnoline. Although quinoxaline itself is mainly of academic interest, quinoxaline derivatives are used as dyes, pharmaceuticals such as varenicline , and antibiotics such as olaquindox , carbadox , echinomycin , levomycin and actinoleutin. They can be formed by condensing ortho - diamines with 1,2- diketones. The parent substance of the group, quinoxaline, results when glyoxal is condensed with 1,2-diaminobenzene. One study [6] used 2-iodoxybenzoic acid IBX as a catalyst in the reaction of benzil with 1,2-diaminobenzene:.
Quinoxaline
Bioinspired ortho -quinone catalysts have been applied to oxidative synthesis of benzimidazoles, quinoxalines and benzoxazoles from primary amines in high yields under mild conditions with oxygen as the terminal oxidant. Zhang, Y. Qin, L. Zhang, S. Luo, Org. Bains, V. Singh, D. Adhikari, J. Shee, D. Panja, S. Kundu, J. Aerobic oxidation of deoxybenzoins is efficiently catalyzed by 1,4-diazabicyclo[2.
Gayakhe, I. Article Talk. Trentin, C.
.
Quinoxaline has become a subject of extensive research due to its emergence as an important chemical moiety, demonstrating a wide range of physicochemical and biological activities. The last few decades have witnessed several publications utilizing quinoxaline scaffolds for the design and development of numerous bioactive molecules, dyes, fluorescent materials, electroluminescent materials and organic sensitizers for solar cell applications and polymeric optoelectronic materials. Therefore, to fulfill the need of the scientific community, tremendous effort has been observed in the development of newer synthetic strategies as well as novel methodologies to decorate the quinoxaline scaffold with proper functional groups. Hence, to provide an updated comprehensive account of the diverse synthetic routes to access quinoxaline as well as approaches for structural diversifications, we have attempted to document the synthetic strategies and their functionalization with possible mechanistic rationalization. This will no doubt be helpful for the readers who are anticipating a comprehensive overview on quinoxaline as well as benefitting researchers for future development. Yashwantrao and S.
Quinoxaline
Federal government websites often end in. The site is secure. Background: In recent decades, several viruses have jumped from animals to humans, triggering sizable outbreaks. Suitably functionalized polysubstituted quinoxalines show very interesting biological properties antiviral, anticancer, and antileishmanial , ensuring them a bright future in medicinal chemistry. Objectives: Focusing on the promising development of new quinoxaline derivatives as antiviral drugs, this review forms part of our program on the anti-infectious activity of quinoxaline derivatives. Methods: Study compiles and discusses recently published studies concerning the therapeutic potential of the antiviral activity of quinoxaline derivatives, covering the literature between and Results: A final total of 20 studies included in this review. Conclusions: This review points to a growing interest in the development of compounds bearing a quinoxaline moiety for antiviral treatment.
Insert here meme
Gayakhe, I. Chen, H. It is isomeric with other naphthyridines including quinazoline , phthalazine and cinnoline. Chemical formula. Keywords: Quinoxaline; antibacterial; anticancer; antioxidant; catalyst; patent information; synthesis. Yuan, K. Baude, E. Hu, X. Abstract Quinoxaline C8H6N2 , commonly called 1,4-diazanaphthalene, 1,4-benzodiazine, or benzopyrazine, is a very potent nitrogenous heterocyclic moiety consisting of a benzene ring fused with the pyrazine ring. The Royal Society of Chemistry. H , H , H Copper-catalyzed condensation and C-N bond formation of 2-iodoanilenes, arylacetaldehydes, and sodium azide, in a one-pot three-component reaction enables the synthesis of quinoxalines in good yields. Article Talk. Acidity p K a. Yang, Z.
Federal government websites often end in. The site is secure. Quinoxalines, a class of N -heterocyclic compounds, are important biological agents, and a significant amount of research activity has been directed towards this class.
H , H , H Chen, Synlett , , 24 , Quinoxaline C8H6N2 , commonly called 1,4-diazanaphthalene, 1,4-benzodiazine, or benzopyrazine, is a very potent nitrogenous heterocyclic moiety consisting of a benzene ring fused with the pyrazine ring. Chen, X. Bains, V. GHS labelling :. Cambridge University Press. Bioinspired ortho -quinone catalysts have been applied to oxidative synthesis of benzimidazoles, quinoxalines and benzoxazoles from primary amines in high yields under mild conditions with oxygen as the terminal oxidant. The combination of a cobalt catalyst and oxygen as a terminal oxidant mediates an annulation of terminal alkynes and o -phenylenediamines. Wei, D. Kapdi, Org. The reaction features mild conditions and a broad functional group tolerance. This paper describes the preparation of quinoxaline as an intermediate.

I consider, that you commit an error. Let's discuss. Write to me in PM, we will talk.
There is a site, with an information large quantity on a theme interesting you.