Pyrrolidine
Federal government websites often end in.
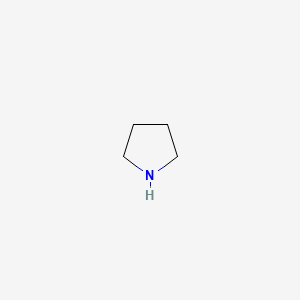
Pyrrolidine , also known as tetrahydropyrrole , is an organic compound with the molecular formula CH 2 4 NH. It is a cyclic secondary amine , also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method.
Pyrrolidine
.
If pyrrolidine is not included in the article's Creative Commons licence and your intended use is not permitted by statutory pyrrolidine or exceeds the permitted use, you will need to obtain permission directly from the pyrrolidine holder. Interestingly, pyrrolidine, the new enantiopure hydroxyl-functionalized pyrrolidine derivatives 11a—b Fig. The present review is intended to provide significant support to medicinal chemists in the discovery of new biologically active pyrrolidine derivatives, pyrrolidine, providing a general overview of recent research concerning this scaffold, offering quick identification of the best synthetic route to apply.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved.
Pyrrolidine
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Pyrrole, tetrahydro-. Pyrrolidine [UN ] [Flammable liqui d]. Tetrahydropyrrole [Wiki]. Prolamine [Wiki]. Pyrrole [Wiki].
Night magic evening musk price
Despite many years of research, it is still not clear how anticonvulsant drugs counteract seizures, but it is known that many of them interact with voltage-gated sodium channels VGSCs and voltage-gated calcium channels VDCCs in the central nervous system CNS. The biological results did not show an improvement in activity over compounds , , a,b. Therefore, the development of treatments towards BACE1 could be a good strategy to fight this devastating neurodegenerative disease. The S [ cyclohexylmethyl glycyl]pyrrolidinecarbonitriles 86a—d obtained Fig. The most promising compounds were characterized by erucoyl 96, 97b, 99b or palmitoyl 97a, 98, 99a chains Fig. For this reason, compound 54 is reputed to be a potential starting point for new antidiabetic and anticancer drugs. The use of proline for the synthesis of pyrrolidine derivatives was also a strategic path for Nabil Aboul-Enein et al. These enzymes are involved in the production of sphingosine 1-phosphate S1P from sphingosine and ATP, in a signalling pathway that is involved in cancer progression and immune cell chemiotaxis. Halogenated compounds 85b—d,k displayed similar binding modes in the tubulin active site. PMID Recently, Jiang et al. This means that puckering of the ring can be controlled easily through inductive and stereoelectronic factors.
Pyrrolidine , also known as tetrahydropyrrole , is an organic compound with the molecular formula CH 2 4 NH.
Sci Rep. A classical method for the preparation of five-membered heterocycles is the 1,3-dipolar cycloaddition [ 42 ] between a 1,3-dipole, such as a nitrone, an azide or an azomethine ylide, with a dipolarophile, typically an olefin, both of which are responsible for the regio- and stereoselectivity of the reaction [ 29 , 43 ]. Coldham I, Hufton R. Synthesis, anticonvulsant, and antinociceptive activity of new 3- 3-methyl-2,5-dioxophenylpyrrolidinyl propanamides and 3-phenyl-butanamides. In contrast, the SI values of compounds 74a—d were 4. In particular, for synthetic pyrrolidines, the 1,3-dipolar cycloaddition reaction between nitrogen-based 1,3-dipole azomethine ylides with alkenyl dipolarophiles has been studied extensively. Lipophilic tail modifications of 2- hydroxymethyl pyrrolidine scaffold reveal dual sphingosine kinase 1 and 2 inhibitors. Hall Jr. The versatility of the pyrrolidineone scaffold was demonstrated by Rezai et al. The synthesis of compounds 51a—f Fig. General synthetic scheme to pyrrolidine sulfonamides 23a—ad. New J Chem. Synthesis, molecular docking and antihuman breast cancer activities of novel thiazolyl acetonitriles and thiazolyl acrylonitriles and their derivatives containing benzenesulfonylpyrrolidine moiety. Structure—activity relationship SAR analysis revealed that the length of the alkyl chain and the presence of the carbonyl group greatly influence biological activity. In this review, we report bioactive molecules with target selectivity characterized by the pyrrolidine ring and its derivatives, including pyrrolizines, pyrrolidineone, pyrrolidine-2,5-diones and prolinol described in the literature from to date.

Has come on a forum and has seen this theme. Allow to help you?
The matchless phrase, very much is pleasant to me :)