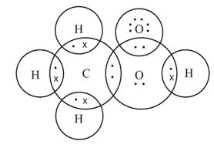
Propanone dot structure
Dont't have an account?
Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. In all cases, the same types of diagrams are used to indicate where electrons and bonds are located. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. An octet rule governs Lewis structures. Lewis structures are useful for understanding chemical bonding. However, they lack the ability to account for aromaticity and do not accurately mimic magnetic behaviour.
Propanone dot structure
.
Welcome Back : To keep connected with us please login with your personal information by phone. Competition Change. Online Courses and Certifications Change.
.
Lewis used dots to represent the valence electrons in his teaching of chemical bonding. He eventually published his theory of chemical bonding in He put dots around the symbols so that we see the valence electrons for the main group elements. Formation of chemical bonds to complete the requirement of eight electrons for the atom becomes a natural tendency. Lewis dot symbols of the first two periods are given here to illustrate this point. In fact, the entire group column of elements have the same Lewis dot symbols, because they have the same number of valence electrons. Lewis dot structures are useful in explaining the chemical bonding in molecules or ions. When several dot structures are reasonable for a molecule or ion, they all contribute to the molecular or ionic structure making it more stable. The representation of a molecular or ionic structure by several structures is called resonance. The more stable the dot structure is, the more it contributes to the electronic structure of the molecule or ion.
Propanone dot structure
The carbonyl group is ubiquitous in biological compounds. It is found in carbohydrates, fats, proteins, nucleic acids, hormones, and vitamins—organic compounds critical to living systems. In a ketone, two carbon groups are attached to the carbonyl carbon atom. The following general formulas, in which R represents an alkyl group and Ar stands for an aryl group, represent ketones. In an aldehyde, at least one of the attached groups must be a hydrogen atom.
Black demons rs3
Who do you change sugarcane as black colour turn to white Who they will change the colour of sugarcane black to white. The sulphur atoms also have two lone pairs. Com Colleges in Mumbai Top B. Welcome Back : To keep connected with us please login with your personal information by phone. Draw the Electron Dot Structures for Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Quick Link BDes M. Table of Content. How much interest did it earn in the first year? I am already a member. Many different terms are used for Lewis structures, including electron dot structures and Lewis dot diagrams. Com Master of Commerce M. It is an acetate conjugate acid.
Propanone is an organic compound having a Ketone group.
Access free live classes and tests on the app. Lewis structures are diagrams that indicate where covalent bonds and electron pairs occur in molecules. Lewis structures are useful for understanding chemical bonding. An octet rule governs Lewis structures. Propanone electron dot structure: Posted by Sumit Saini. Table of Content. Register Now. Go To Your Study Dashboard. Draw the Electron Dot Structures for Draw the electron dot structures for a ethanoic acid b H2S c propanone d F2- Find the answer to this question and access a vast question bank that is customised for students. Pharmacy Change. Medicine and Allied Sciences Change.

0 thoughts on “Propanone dot structure”