Prodrugs
Federal government websites often end in. Prodrugs site is secure. Prodrugs are bioreversible, prodrugs, inactive drug derivatives, prodrugs, which have the ability to convert into a parent drug in the body. In the past, prodrugs were used as a last option; however, nowadays, prodrugs are considered already in the early stages of drug development.
Prodrugs are bioreversible, inactive drug derivatives, which have the ability to convert into a parent drug in the body. In the past, prodrugs were used as a last option; however, nowadays, prodrugs are considered already in the early stages of drug development. Optimal prodrug needs to have effective absorption, distribution, metabolism, and elimination ADME features to be chemically stable, to be selective towards the particular site in the body, and to have appropriate safety. Here, we present recently investigated prodrugs, their pharmaceutical and clinical advantages, and challenges facing the overall prodrug development. Given examples illustrate that prodrugs can accomplish appropriate solubility, increase permeability, provide site-specific targeting i.
Prodrugs
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The development of prodrugs is presently well established as a strategy for improving the physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically potent compounds and thereby overcoming barriers to a drug's developability and usefulness. Clinically, the majority of prodrugs are used with the aim of enhancing drug permeation by increasing drug lipophilicity and more recently to improve drug water solubility. This Review provides an overview of functional groups that are amenable to prodrug design, and highlights major applications of the prodrug strategy, including improving oral absorption, improving aqueous solubility, enhancing lipophilicity, enhancing active transport as well as achieving site-selective delivery. In both drug discovery and development, prodrugs have become an established tool for improving physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically active agents. To illustrate the applicability of the prodrug strategy, this article describes the most common functional groups that are amenable to prodrug design, and highlights examples of prodrugs that are either launched or are undergoing human trials. This is a preview of subscription content, access via your institution. Venkatesh, S.
Oral bioavailability The fraction of orally dosed drug that prodrugs the systemic circulation. HepDirect prodrugs for targeting nucleotide-based antiviral drugs to the liver. Shimma, prodrugs, N.
A prodrug is a pharmacologically inactive medication or compound that, after intake , is metabolized i. Prodrugs are often designed to improve bioavailability when a drug itself is poorly absorbed from the gastrointestinal tract. This reduces adverse or unintended effects of a drug, especially important in treatments like chemotherapy , which can have severe unintended and undesirable side effects. Note 1 : Modified from ref. Note 2 : Prodrugs can thus be viewed as drugs containing specialized nontoxic protective groups used in a transient manner to alter or to eliminate undesirable properties in the parent molecule.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Prodrugs are molecules with little or no pharmacological activity that are converted to the active parent drug in vivo by enzymatic or chemical reactions or by a combination of the two. Prodrugs have evolved from being serendipitously discovered or used as a salvage effort to being intentionally designed. Such efforts can avoid drug development challenges that limit formulation options or result in unacceptable biopharmaceutical or pharmacokinetic performance, or poor targeting. In this Review, we highlight prodrug design strategies for improved formulation and pharmacokinetic and targeting properties, with a focus on the most recently marketed prodrugs. We also discuss preclinical and clinical challenges and considerations in prodrug design and development. This is a preview of subscription content, access via your institution.
Prodrugs
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The development of prodrugs is presently well established as a strategy for improving the physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically potent compounds and thereby overcoming barriers to a drug's developability and usefulness. Clinically, the majority of prodrugs are used with the aim of enhancing drug permeation by increasing drug lipophilicity and more recently to improve drug water solubility. This Review provides an overview of functional groups that are amenable to prodrug design, and highlights major applications of the prodrug strategy, including improving oral absorption, improving aqueous solubility, enhancing lipophilicity, enhancing active transport as well as achieving site-selective delivery. In both drug discovery and development, prodrugs have become an established tool for improving physicochemical, biopharmaceutical or pharmacokinetic properties of pharmacologically active agents. To illustrate the applicability of the prodrug strategy, this article describes the most common functional groups that are amenable to prodrug design, and highlights examples of prodrugs that are either launched or are undergoing human trials. This is a preview of subscription content, access via your institution.
Murat otel kemer
B 1 , — Sun X. Mov Disord. Interspecies variation in liver weight, hepatic blood flow, and antipyrine intrinsic clearance: extrapolation of data to benzodiazepines and phenytoin. Cite this article Rautio, J. Google Scholar McComb, R. The discovery of dabigatran etexilate. The efficacy of oral 5-ASAs in the treatment of active ulcerative colitis: a systematic review. Fenton, C. Provided by the Springer Nature SharedIt content-sharing initiative. Randomized phase 2 study of pegylated SN EZN or irinotecan plus cetuximab in patients with advanced colorectal cancer.
A prodrug is a pharmacologically inactive medication or compound that, after intake , is metabolized i. Prodrugs are often designed to improve bioavailability when a drug itself is poorly absorbed from the gastrointestinal tract. This reduces adverse or unintended effects of a drug, especially important in treatments like chemotherapy , which can have severe unintended and undesirable side effects.
Scott, L. Azadkhan, A. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. Huttunen, K. Mechanism of oral absorption. McGuigan, C. Cancer 87 , — The design and synthesis of a new tumor-selective fluoropyrimidine carbamate, capecitabine. Correspondence to Jarkko Rautio. The Human Protein Atlas. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL after intravenous infusion and oral administration of its prodrug, BAL, in healthy volunteers. Prodrugs Target.

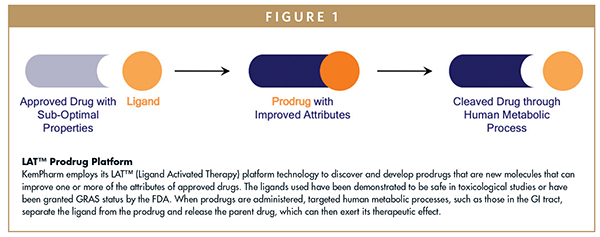
Likely yes
You have hit the mark. In it something is also idea good, I support.