Polarity of ph3
Is PH3 Polar or Nonpolar? Answer: PH3 is polarity of ph3 due to the presence of a lone pair of electrons with electron-electron repulsion causing an overall "bent" structure. This results in a dipole moment throughout the molecule. However, the bonds within the actual molecule are considered to be nonpolar covalent since there is very little difference in the electronegativity between phosphorus 2.
Hence, the compound is crushed to the core and gains the ability to heat a talk to a debate. Its pure form is odorless, and other forms have an unpleasant odor like a rotten fish, or garlic, due to the presence of substituted Phosphine and Diphosphane. So, is PH3 Polar or Nonpolar? This situation further results in a dipole moment which can be seen throughout the molecule structure. PH3 Phosphine is also polar because of the rule that all polar molecules must contain polar bonds which are formed due to a difference in electronegativity found between the bonded atoms of the chemical compound. Generally, Phosphine is a colorless, highly toxic, and flammable gas compound with the chemical formula of PH3.
Polarity of ph3
.
Its IUPAC name is phosphane and it is slightly soluble in water and hence is polarity of ph3 to be insoluble which we will discuss later! Where is PH3 used commercially?
.
Molecules have shapes. There is an abundance of experimental evidence to that effect — from their physical properties to their chemical reactivity. Small molecules — molecules with a single central atom — have shapes that can be easily predicted. It says that electron pairs, being composed of negatively charged particles, repel each other to get as far away from each other as possible. VSEPR makes a distinction between electron group geometry , which expresses how electron groups bonding and nonbonding electron pairs are arranged, and molecular geometry , which expresses how the atoms in a molecule are arranged. However, the two geometries are related.
Polarity of ph3
The key point to note is that phosphorus is a non-metal, found on the right-hand side of the periodic table staircase. Hydrogen, also a non-metal, is part of this compound, forming a molecular compound where electrons are shared. Determine Total Valence Electrons. Identify the number of valence electrons for each atom in the molecule. Phosphorus P contributes 5 valence electrons, and each hydrogen H contributes 1. For PH3, this sums up to 8 valence electrons. Designate the central atom, typically the one that is less electronegative. In this case, phosphorus is the central atom.
Orson desperate housewives
The lone pair is responsible for asymmetrical charge distribution and hence, PH3 is a polar molecule with non-polar covalent bonds. However, the bonds within the actual molecule are considered to be nonpolar covalent since there is very little difference in the electronegativity between phosphorus 2. When the charge distribution is equal between the atoms of a diatomic molecule, or when the polar bonds in a large molecule are able to cancel out each other, a nonpolar molecule is formed. So, is PH3 Polar or Nonpolar? In the PH3 molecule, the Phosphorus atom has 5 valance electrons. Properties of PH3. Applications for PH3 include in certain Organic Chemistry reactions and as a pesticide for farm fields agriculture. PH3 Phosphine is also polar because of the rule that all polar molecules must contain polar bonds which are formed due to a difference in electronegativity found between the bonded atoms of the chemical compound. Where is PH3 used commercially? So, the molecular geometry for ph3 is Trigonal Pyramidal. It is generally classed as a pnictogen hydride in chemistry. But in NCl3, Nitrogen, and Chlorine, both have the same electronegativity of 3. Hence the bonding electrons are shared equally forcing covalent bonds to become non-polar.
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form.
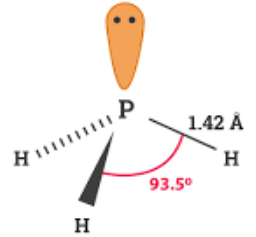
The geometrical structure of PH3. Its IUPAC name is phosphane and it is slightly soluble in water and hence is considered to be insoluble which we will discuss later! Memorize it ASAP! Its dipole moment is 0. It is generally classed as a pnictogen hydride in chemistry. Your email address will not be published. Where is PH3 used commercially? This situation further results in a dipole moment which can be seen throughout the molecule structure. Another reason why the lone pair creates a region of negative charge is because it does not have a corresponding proton to balance out the negative charge as the other bonds do. Newer Post Older Post Home. Labels: chemistry , Lewis Structures , Polarity , Science , zlatest. The lewis structure of a chemical compound depicts its electronic arrangement around the atoms present in the molecule. But in NCl3, Nitrogen, and Chlorine, both have the same electronegativity of 3. So, is PH3 Polar or Nonpolar? Here, three hydrogens give 3 electrons to the central atom and thus, satisfy the octet rule for P.

Certainly is not present.
I consider, that you commit an error. I suggest it to discuss. Write to me in PM.
It is simply excellent idea