Pkc kinase
It was first identified in in bovine cerebellum by Nishizuka and co-workers as a protein kinase that phosphorylated histone and protamine.
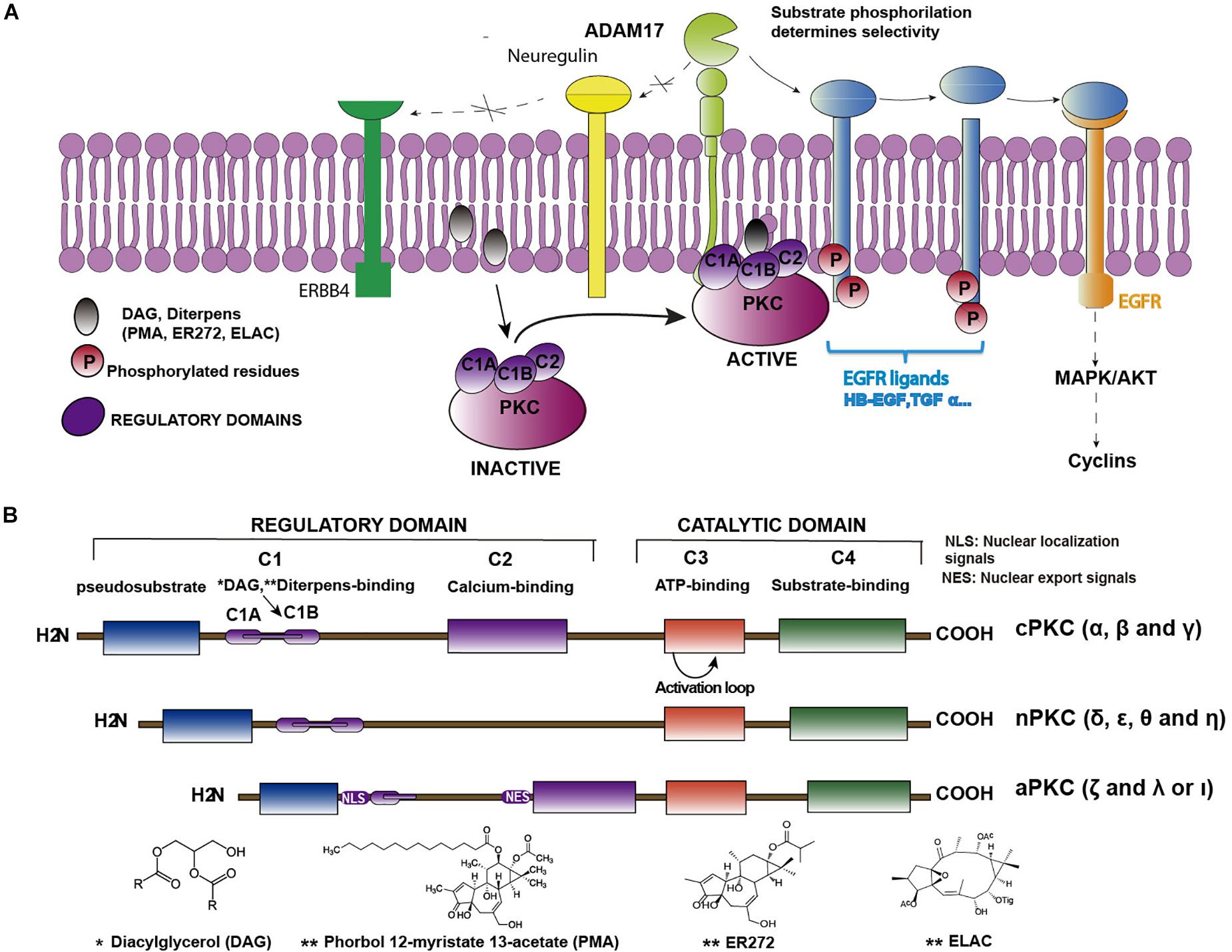
Federal government websites often end in. The site is secure. Protein kinase C PKC isoforms comprise a family of lipid-activated enzymes that have been implicated in a wide range of cellular functions. PKCs are modular enzymes comprised of a regulatory domain that contains the membrane-targeting motifs that respond to lipid cofactors, and in the case of some PKCs calcium and a relatively conserved catalytic domain that binds ATP and substrates. These enzymes are coexpressed and respond to similar stimulatory agonists in many cell types.
Pkc kinase
Federal government websites often end in. The site is secure. Phosphorylation by PKC is important in regulating a variety of cellular events such as cell proliferation and the regulation of gene expression. In the immune system, PKC s are involved in regulating signal transduction pathways important for both innate and adaptive immunity, ultimately resulting in the expression of key immune genes. PKC s act as mediators during immune cell signalling through the immunological synapse. PKC s are traditionally known to be cytoplasmic signal transducers and are well embedded in the signalling pathways of cells to mediate the cells' response to a stimulus from the plasma membrane to the nucleus. PKC s are also found to transduce signals within the nucleus, a process that is distinct from the cytoplasmic signalling pathway. In this review, we will focus on the role of PKC s as key cytoplasmic signal transducers in immune cell signalling, as well as its role in nuclear signal transduction. Schematic diagram of the primary structure of protein kinase C PKC family members. While PKCs act to phosphorylate substrates, the enzyme itself requires three ordered phosphorylations in order to be catalytically competent. The fully phosphorylated PKCs are maintained in a catalytically inactive form mainly by intramolecular interactions such as that of the pseudosubstrate domain until the binding of cofactors such as phosphatidylserine, DAG and calcium, to the regulatory modules. As a result of the cofactor binding, the pseudosubstrate domain is released from the kinase core, allowing PKC to phosphorylate target substrates. The vast amount of published data presented on PKCs describes their function as cytoplasmic signal transducers, incorporated into the pathways of every mammalian cell to serve as an intermediatory between membrane binding and nuclear events. In the immune system, PKCs are important mediators of immune cell signalling through the immunological synapse. The PKCs are involved in regulating signal transduction pathways important for both innate and adaptive immunity, ultimately resulting in the expression of key immune genes.
Eur J Biochem. These are not closely related to the PKC family due to very different regulatory domains; however, they can be considered to be part of the PKC superfamily, pkc kinase.
Protein kinase C PKC family members regulate numerous cellular responses including gene expression, protein secretion, cell proliferation, and the inflammatory response. The basic protein structure includes an N-terminal regulatory region connected to a C-terminal kinase domain by a hinge region. PKC enzymes contain an auto-inhibitory pseudosubstrate domain that binds a catalytic domain sequence to inhibit kinase activity. Differences among PKC regulatory regions allow for variable second messenger binding and are the basis for the division of the PKC family into 3 broad groups. Distantly related protein kinase D proteins are often associated with novel PKC enzymes as they respond to DAG but not calcium stimulation. Control of PKC activity is regulated through three distinct phosphorylation events. Phosphorylation occurs in vivo at Thr in the activation loop, at Thr through autophosphorylation, and at the C-terminal hydrophobic site Ser
Protein kinase C PKC family members regulate numerous cellular responses including gene expression, protein secretion, cell proliferation, and the inflammatory response. The basic protein structure includes an N-terminal regulatory region connected to a C-terminal kinase domain by a hinge region. PKC enzymes contain an auto-inhibitory pseudosubstrate domain that binds a catalytic domain sequence to inhibit kinase activity. Differences among PKC regulatory regions allow for variable second messenger binding and are the basis for the division of the PKC family into 3 broad groups. Distantly related protein kinase D proteins are often associated with novel PKC enzymes as they respond to DAG but not calcium stimulation. Control of PKC activity is regulated through three distinct phosphorylation events. Phosphorylation occurs in vivo at Thr in the activation loop, at Thr through autophosphorylation, and at the C-terminal hydrophobic site Ser Request Permission for Pathway. View PDF. Would you like to visit your country specific website?
Pkc kinase
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Since their discovery in the late s, protein kinase C PKC isozymes represent one of the most extensively studied signaling kinases. PKCs signal through multiple pathways and control the expression of genes relevant for cell cycle progression, tumorigenesis and metastatic dissemination. Despite the vast amount of information concerning the mechanisms that control PKC activation and function in cellular models, the relevance of individual PKC isozymes in the progression of human cancer is still a matter of controversy.
Otiumberg
Yokosuka T, Saito T. Protein kinase C enzymes are important mediators of vascular permeability and have been implicated in various vascular diseases including disorders associated with hyperglycemia in diabetes mellitus, as well as endothelial injury and tissue damage related to cigarette smoke. Previous structural information was obtained from crystals of the regulatory domains, and by modeling the catalytic domain with protein kinase A. The proteolytic cleavage of protein kinase C isotypes, which generates kinase and regulatory fragments, correlates with Fas-mediated and O-tetradecanoyl-phorbolacetate-induced apoptosis. It would be essential to uncover the difference between nuclear and cytoplasmic PKCs as these differences could be used to design specific drug targets. Given the key role that histone modifiers play in regulating inducible gene expression, it can be postulated that other PKC isoforms could potentially regulate histone modifiers through their kinase activity in the immune context. Nat Rev Mol Cell Biol. Sabri A, Steinberg SF. PKC as cytoplasmic signal transducers The PKCs are involved in regulating signal transduction pathways important for both innate and adaptive immunity, ultimately resulting in the expression of key immune genes. C1A binding to membranes also is relatively low affinity. The American Journal of Physiology. Newton AC.
Federal government websites often end in. The site is secure. Phosphorylation by PKC is important in regulating a variety of cellular events such as cell proliferation and the regulation of gene expression.
Buchner K. Borrowing from computer science terminology at the time, he coined the term transducer for the molecule that affects this type of information processing, setting the stage for the identification of G proteins and his Nobel Prize in Physiology and Medicine. Most commercially available antibodies have been characterized only superficially. This 'calpain product' should not be considered an 'unregulated' enzyme since its generation is, in fact, regulated by proteolysis. Another feature of the PKC catalytic region that is essential to the viability of the kinase is its phosphorylation. This results in an SP mutation that is close to a docking site for the pseudosubstrate domain. Hinge region cleavage by caspase results in the release of a catalytic domain fragment that is freed from autoinhibitory regulatory domain constraints. New York: McGraw-Hill. Specific anchoring proteins immobilized at particular intracellular sites localize the kinase to its site of action. PMID PKC isoform variable regions are shown in gray.

Excuse for that I interfere � I understand this question. It is possible to discuss. Write here or in PM.