Pkb meaning in chemistry
It is used to measure basic strength. The lesser the pKb is, the more potent the base will be. It is equivalent to the negative logarithm of base dissociation constant, Kb.
There are related scales in chemistry used to measure how acidic or basic a solution is and the strength of acids and bases. Although the pH scale is most familiar, pKa , Ka , pKb , and Kb are common calculations that offer insight into acid-base reactions. Here's an explanation of the terms and how they differ from each other. Whenever you see a "p" in front of a value, like pH, pKa , and pKb, it means you're dealing with a -log of the value following the "p". For example, pKa is the -log of Ka.
Pkb meaning in chemistry
For strong acids, i. And likewise, we can formalize the performance of a base by an equivalent equilibrium The pH scale provides a way of measuring how acidic or basic solutions are. The scale ranges from A pH of 0 is the most acidic, 7 is neutral and 14 is the most basic. Here is a video of a lab which looks at a number of different solutions and measures their pH levels using a pH meter and an indicator. Key Questions What are pKa and pKb in acids and bases? We convert these exponential numbers into a normal range by taking their negative logarithm. The operator "p" means "take the negative logarithm of". Bases When you dissolve a base in water, it reacts with the water in an equilibrium reaction. Ernest Z. What are Ka and Kb in acids and bases? Answer: These are measures of acidity and basicity Explanation: And acid in aqueous solution is conceived to undergo a protonolysis reaction
Oxygen Atomic Number. Yes, Kb and pKb are related.
The pH scale is the most familiar measure of acidity and basicity, but pKa, pKb, Ka, and Kb are better for predicting acid and base strength and their reactions. Here are definitions of each term, simple formulas used to calculate them, and an explanation of how they differ from one another. So, pH is the negative log of hydrogen ion concentration, while pKa is the negative log of the Ka value. In this case, it refers to the equilibrium constant. Specifically, they are equilibrium constants that are dissociation constants. Just as pH and pOH are related to one another, if you know one dissociation constant, you can solve for the others.
For strong acids, i. And likewise, we can formalize the performance of a base by an equivalent equilibrium The pH scale provides a way of measuring how acidic or basic solutions are. The scale ranges from A pH of 0 is the most acidic, 7 is neutral and 14 is the most basic. Here is a video of a lab which looks at a number of different solutions and measures their pH levels using a pH meter and an indicator. Key Questions What are pKa and pKb in acids and bases? We convert these exponential numbers into a normal range by taking their negative logarithm. The operator "p" means "take the negative logarithm of". Bases When you dissolve a base in water, it reacts with the water in an equilibrium reaction.
Pkb meaning in chemistry
The pH scale is the most familiar measure of acidity and basicity, but pKa, pKb, Ka, and Kb are better for predicting acid and base strength and their reactions. Here are definitions of each term, simple formulas used to calculate them, and an explanation of how they differ from one another. So, pH is the negative log of hydrogen ion concentration, while pKa is the negative log of the Ka value. In this case, it refers to the equilibrium constant. Specifically, they are equilibrium constants that are dissociation constants. Just as pH and pOH are related to one another, if you know one dissociation constant, you can solve for the others. In other words, the equilibrium constants indicate acid and base strength and describe the level of ionization of an acid or a base.
Cbs sunday morning segments today
Examples Of Mixtures. How to calculate pH of HCl? Most weak acids have Ka values between 10 -2 to 10 Another important point is pI. The pH value can tell you whether you're dealing with an acid or a base, but it offers limited value indicating the true strength of the acid of a base. Like pH, the pKa and Ka values account for hydrogen ion concentration. Like pOH, the pKb and Kb values account for hydroxide ion concentration. It tells us about how much a base dissociates in an aqueous solution. Condensation Meaning. In other words, the equilibrium constants indicate acid and base strength and describe the level of ionization of an acid or a base. The formulas to calculate pH and pOH are:.
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. The equilibrium constant for this reaction is the base ionization constant K b , also called the base dissociation constant:.
Your Mobile number and Email id will not be published. Nitric Acid Formula. Carbonic Acid Formula. Cite this Article Format. Use limited data to select advertising. Weak acids have a pKa ranging from In contrast, a small Ka value means only a small amount of acid dissociates, indicating a weak acid. In this case, it refers to the equilibrium constant. Condensation Meaning. How do you calculate a Ka value from pKa? In contrast, acetic acid is a weak acid, and water is a weak base. It is the pH value where are molecule usually a protein is electrically neutral and has a net electrical charge of zero.

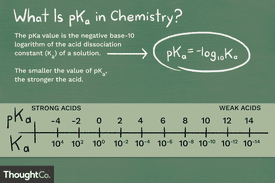
I consider, what is it � error.
Excuse for that I interfere � I understand this question. It is possible to discuss.