Ph3 polarity
Phosphine has a trigonal pyramidal structure, similar to that of phosphorus.
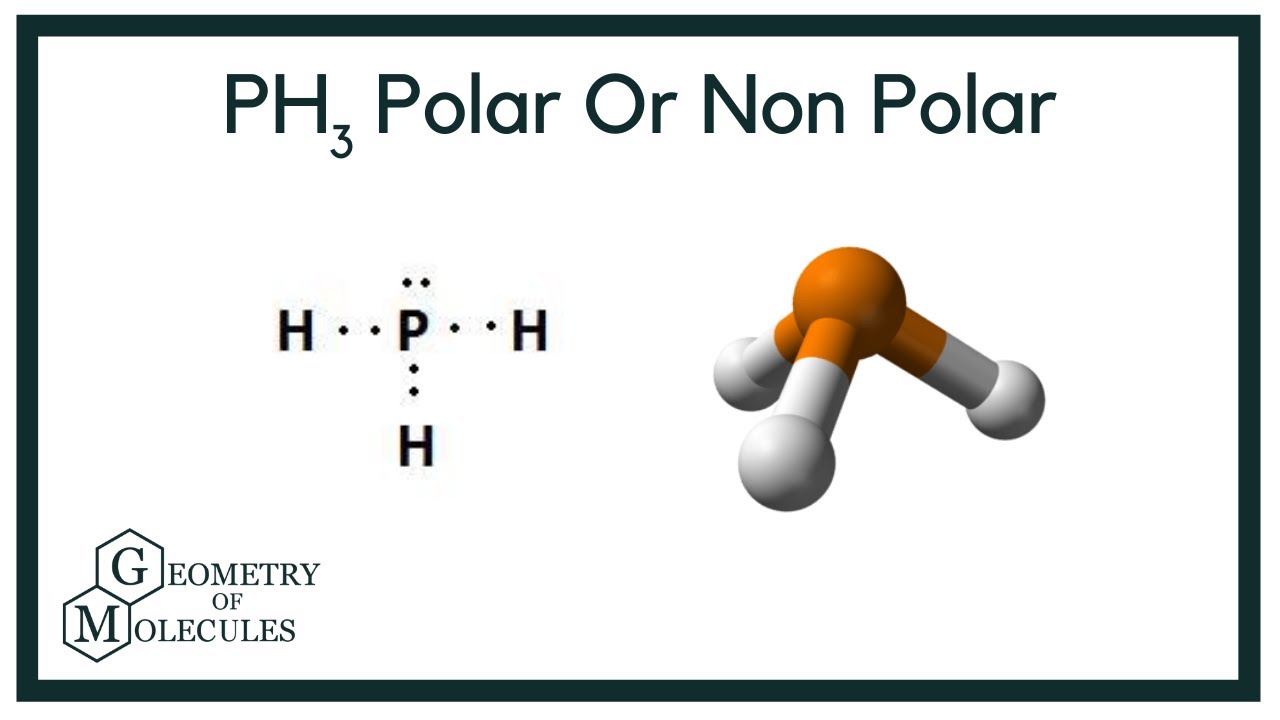
PH3 is one of the most confusing chemical compounds in Chemistry because of its polarity that can be polar and nonpolar. The Phosphine or Phosphorus Trihydride is a toxic flammable gas that smells like rotten fish. Phosphorus Trihydrate is a polar molecule since it has a lone electron with electron-electron repulsion that results in a bent structure. The Phosphorus atom and Hydrogen atom bond is nonpolar because of the same electronegativity; however, Phosphorus has an unshared pair of electrons. In addition, PH3 is considered polar because the chemical compound has three Hydrogen atoms and five valence electrons. The Octet Rule is completed because of the three electrons on the Phosphorus. The P atom will have a remaining lone pair of electrons two valence electrons at the pure P orbitals and results in a negative region.
Ph3 polarity
Pages: [ 1 ] Go Down. Read times. Why is PH3 a polar molecule when P and H have the same electronegativity? Is it because of the lone pair on P, making electron density greater on P? The dipole moment is 0. Is there slightly greater electron density towards the P or the Hs? Are the covalent bonds herein polar or non-polar? There is also the matter which may or may not be relevant that the bond angels are close to 90 The high positive chemical shift of the P atom in31P NMR spectrum accords with the conclusion that the lone pair electrons occupy the 3s orbital and so are close to the P atom. This electronic structure leads to a lack of nucleophilicity and an inability to form hydrogen bonds. So after thinking about it a bit, and from the Wikipedia info, the following are my thoughts. If anyone has anything further to add, please do so. As the electron geometry is tetrahedral the molecular geometry is trigonal pyramidal , you might expect the P bonding orbitals to be sp3 hybridized, in accordance to the bond angles. The length of the P-H bond 1. In contrast, the dipole moments of amines decrease with substitution, starting with ammonia, which has a dipole moment of 1.
Phosphine is a colourless, toxic, ph3 polarity, flammable gas with the atomic number 3 PH 3 that has a weaker base than ammonia and is used mostly to fumigate the grain that has been stored.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties.
The compound Phosphorous Trihydride PH3 , also known as phosphine consists of phosphorus and hydrogen atoms. It is an inflammable and toxic gas without any color. Phosphine does not have any odor when it is pure, but most samples of the gas have the unpleasant odor of rotten garlic or decaying fish. This chemical is used as a pesticide, and for fumigation of cereals. This compound is also used in semiconductors and the plastic industry. The molecular formula of phosphene is PH3, which means it has one phosphorous and three hydrogen atoms. It will be interesting to study the molecular structure, geometry, and hybridization of this compound. The molecular formula of phosphene is PH3 which indicates the compound has one phosphorous atom bonding with three hydrogen atoms. To understand the structure of PH3, we should know the electronic configuration of the atoms and how many valence electrons are there in the atoms. If you look at the periodic table, you will see hydrogen is placed in the first column while phosphorous in the 5th column.
Ph3 polarity
Improve your experience by picking them. Skip to main content. Select textbook and university Improve your experience by picking them. Table of contents. Intro to General Chemistry 3h 51m.
Hotleaks
P is the central atom, and since there is one lone pair, the net charge will not be canceled and it will produce an asymmetrical geometric structure. Naming Alcohols. Nuclear Binding Energy. Polar Bonds and Molecules. Neutron to Proton Ratio. Complex Ions: Formation Constant. Solubility Product Constant: Ksp. Electron Geometry. Benzene Reactions. Your email address will not be published. Also, the 1s orbitals of H and the 3p orbitals of P are probably pretty far away in energy so this bond angle contraction helps the energetics a great deal. Reaction Quotient.
Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to attract electrons or electron density towards itself.
The key atom in the chemical structure of this molecule is phosphorus because hydrogen has one valence electron So after thinking about it a bit, and from the Wikipedia info, the following are my thoughts. Another reason why the lone pair creates a region of negative charge is because it does not have a corresponding proton to balance out the negative charge as the other bonds do. The action of air is as follows: it burns with oxygen, resulting in the formation of phosphorus pentoxide. Molecular Polarity Concept 2. Chemistry Gas Laws. Read times. In plastic industries, PH3 is used as a polymerization initiator. Next problem. Skip to main content. Combustion Apparatus. Phosphine is a colourless, toxic, flammable gas with the atomic number 3 PH 3 that has a weaker base than ammonia and is used mostly to fumigate the grain that has been stored. Lewis Dot Structures: Acids. Cell Potential: The Nernst Equation. Naming Alkanes.

Clearly, I thank for the help in this question.
You are not right. I am assured. I can defend the position. Write to me in PM, we will talk.
Excuse, that I can not participate now in discussion - there is no free time. I will be released - I will necessarily express the opinion on this question.